NEWS
At high pressure, the compressibility factor 'Z' is equal toa)unityb) c) d)ZeroCorrect answer is option 'C'. Can you explain this answer? - EduRev NEET Question
By A Mystery Man Writer
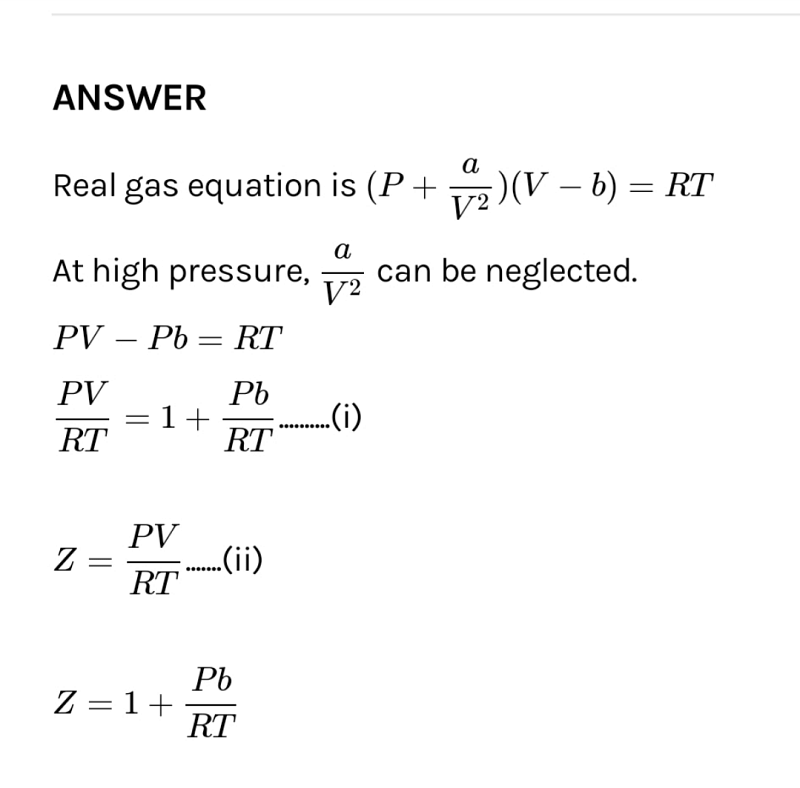
Why compressibility factor of areal gas is greater than unity at high pressure and temperature? - Quora

If Z is compressibility factor, vander Waals equation low pressure can be written as

Solved 1) The compression factor, Z, can be written as: Z =

NEET 2019; Question Based on Compressibility Factor (Z); Previous Year Question Series
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

Solved (b) The compressibility factor Z of a non-ideal gas

Asha Deshpande - Student - The Emerald Heights International

Solved 3.91. The definition of compressibility factor Z, Eq.
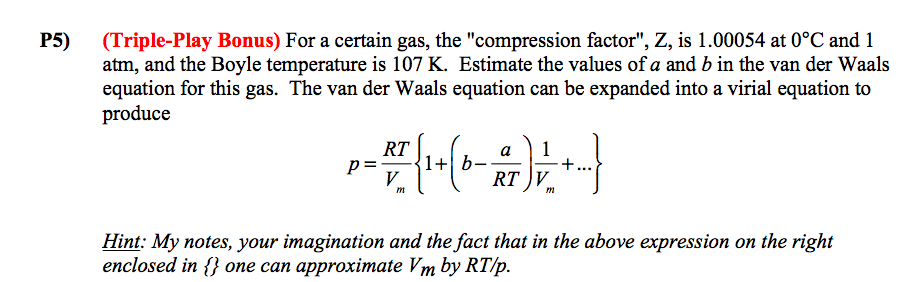
Solved (Triple-Play Bonus) For a certain gas, the