1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts
By A Mystery Man Writer

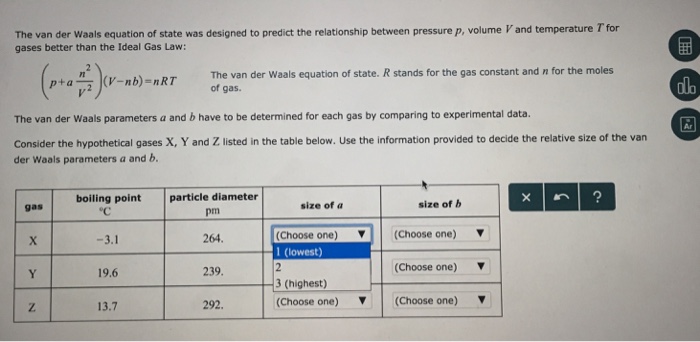
Solved The van der Waals equation of state was designed to
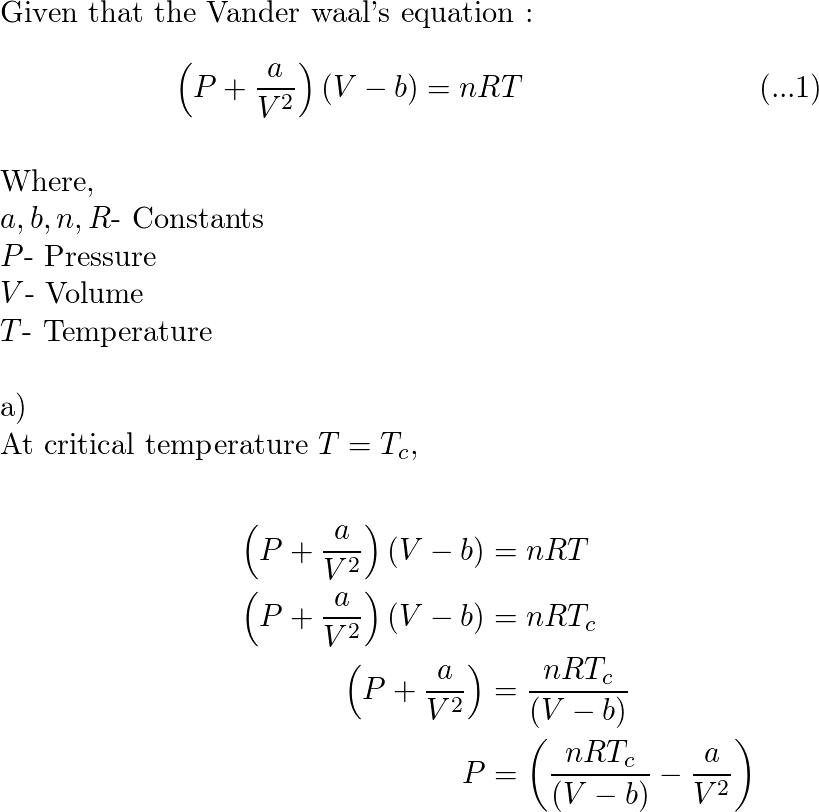
In physical chemistry, it is shown that the pressure P of a
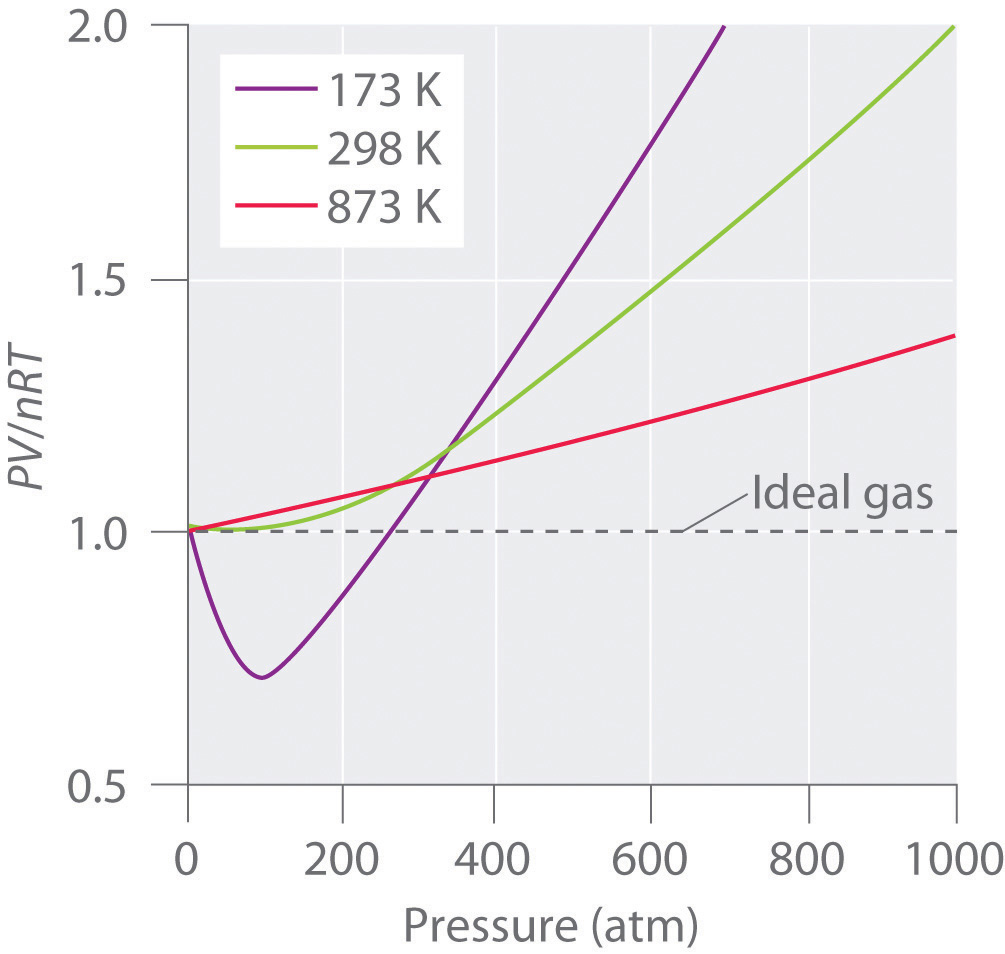
Chapter 11.1: Real Gases - Chemistry LibreTexts

Boyle Temperature and Critical constants, Derivation from Van der Waals equation

Full PDF, PDF, Atomic Nucleus

Van der Waals Equation Practice Problems
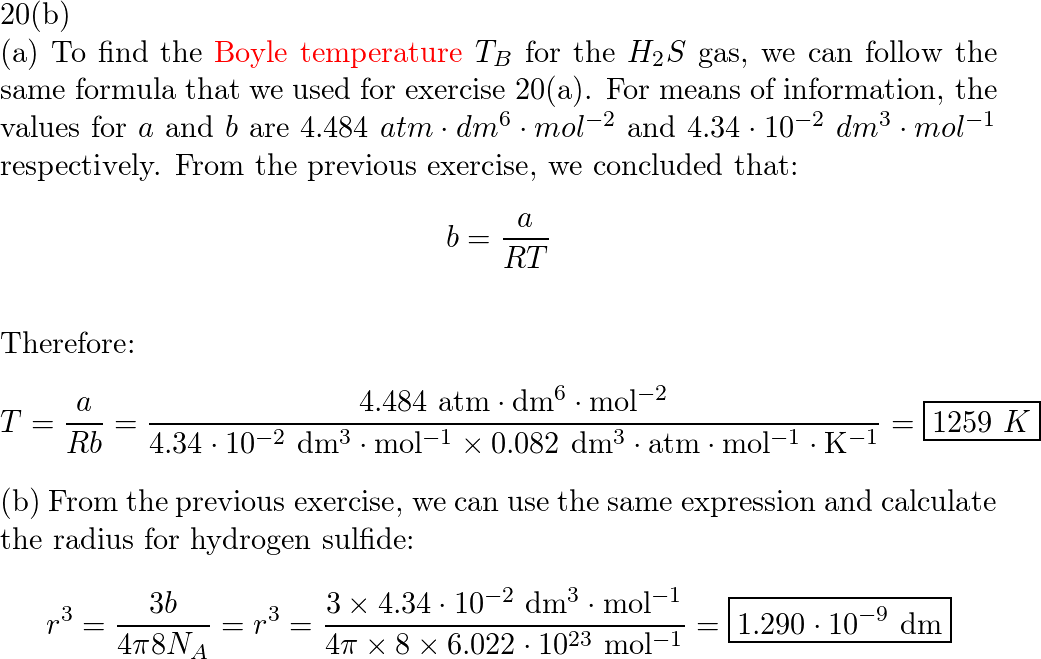
a) Use the van der Waals parameters for chlorine of the Res
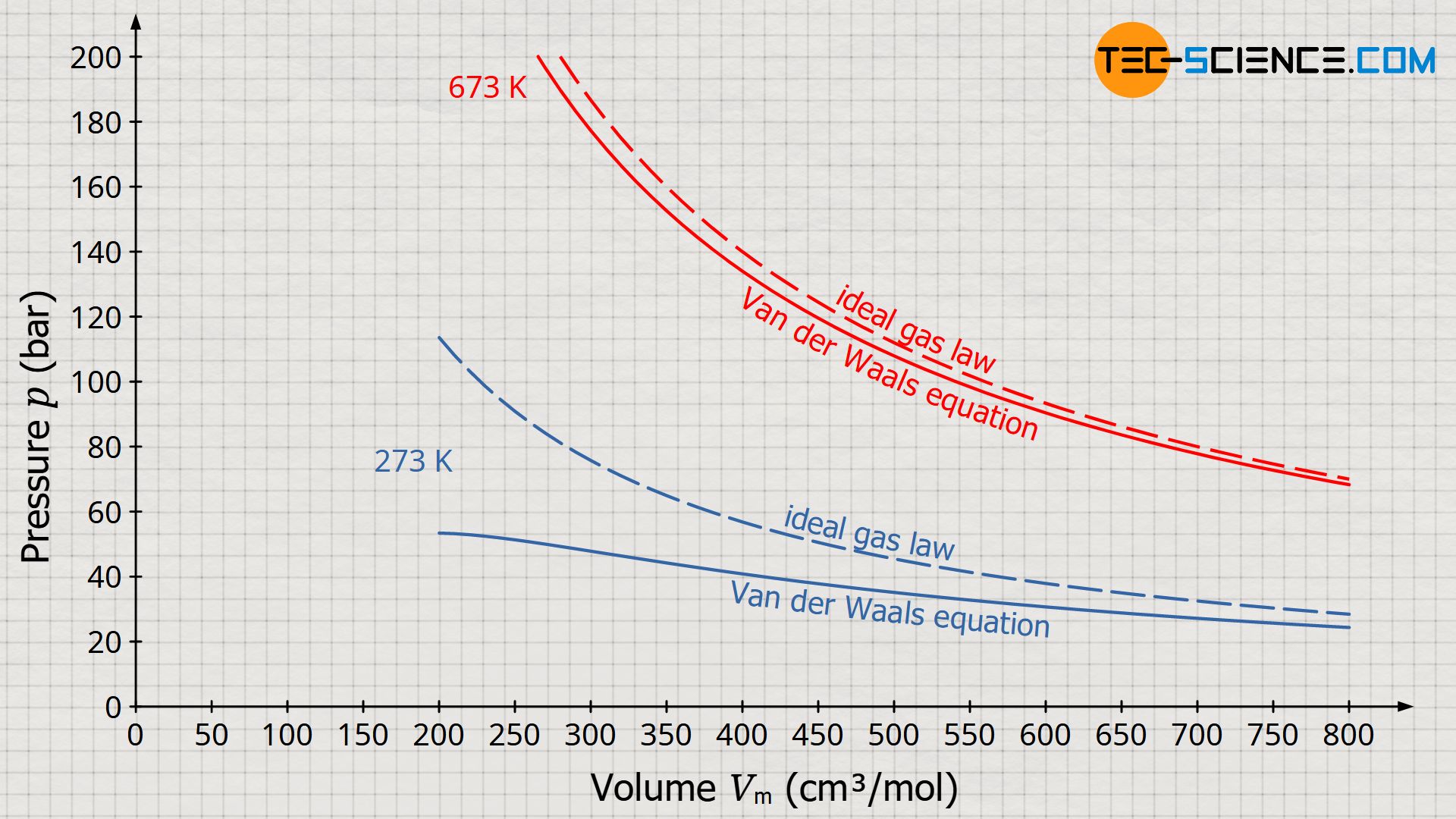
Van der Waals equation (gas law for real gases) - tec-science
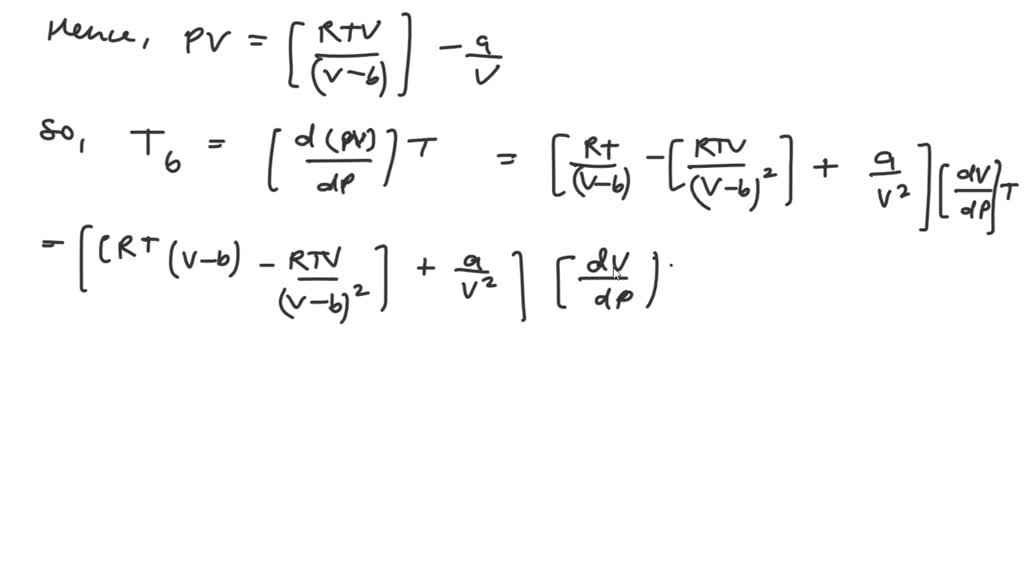
SOLVED: Find the mathematical expression for the Boyle temperature of a van der Waals gas, then find the value of the Boyle temperature of chlorine gas as predicted by the van der
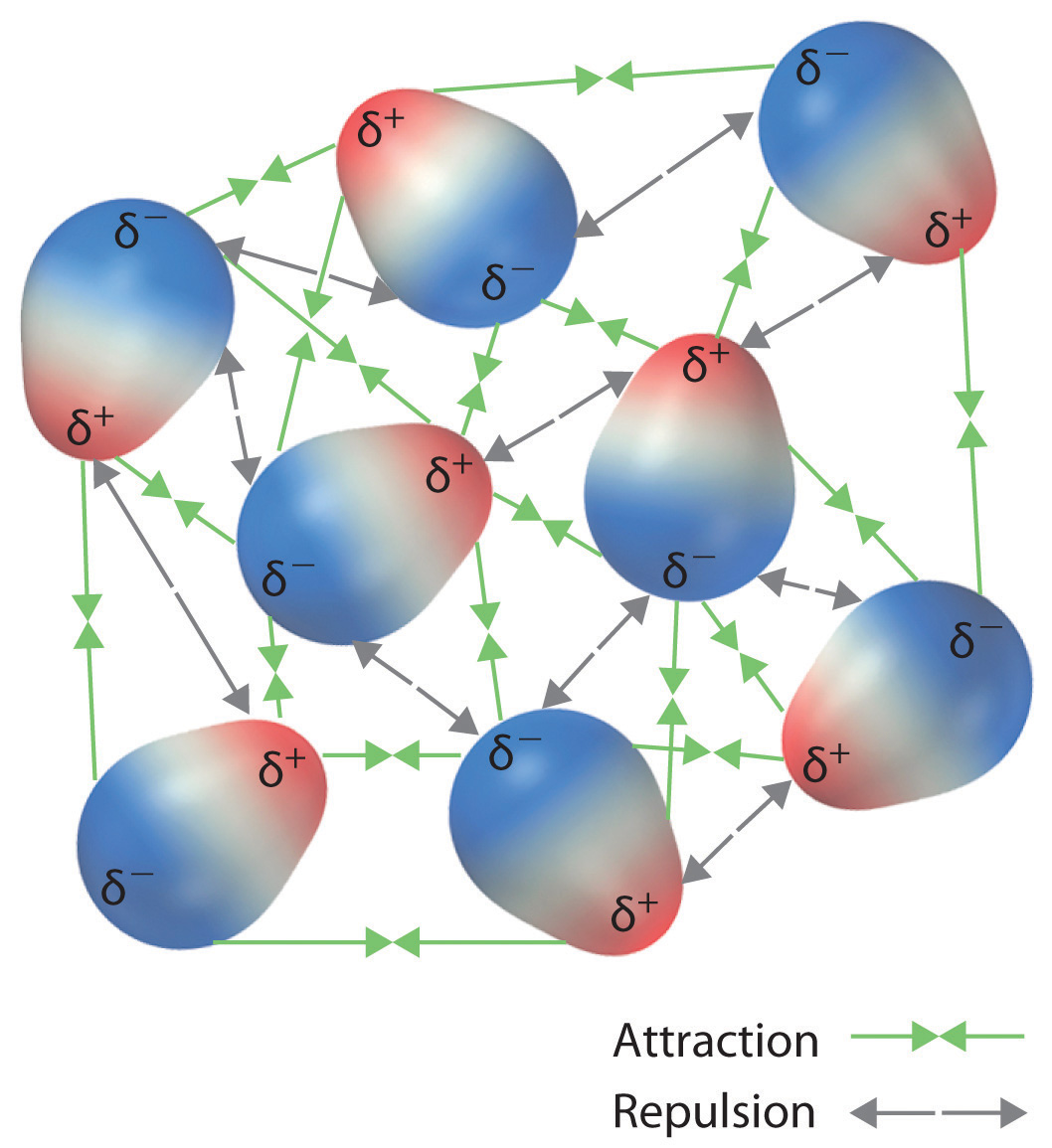
11.2: Intermolecular forces - Chemistry LibreTexts
How do Van der Waals constants a and b depend on temperature, pressure and volume? - Quora
UAH Global Temperature Update for February, 2023: +0.08 deg. C « Roy Spencer, PhD

For a van der Waal's gas, determine Boyle Temperature. [ given mathrm{a}=4.5 mathrm{atm} mathrm{L}^2mathrm{mol}^{-2}, mathrm{b}=0.9 mathrm{L} mathrm{mol}^{-1} and R=0.082 mathrm{L} mathrm{atm} mathrm{K}^{-1} mathrm{mol}^{-1}].609.8K6.09K273K60.98K
Course: Chemistry