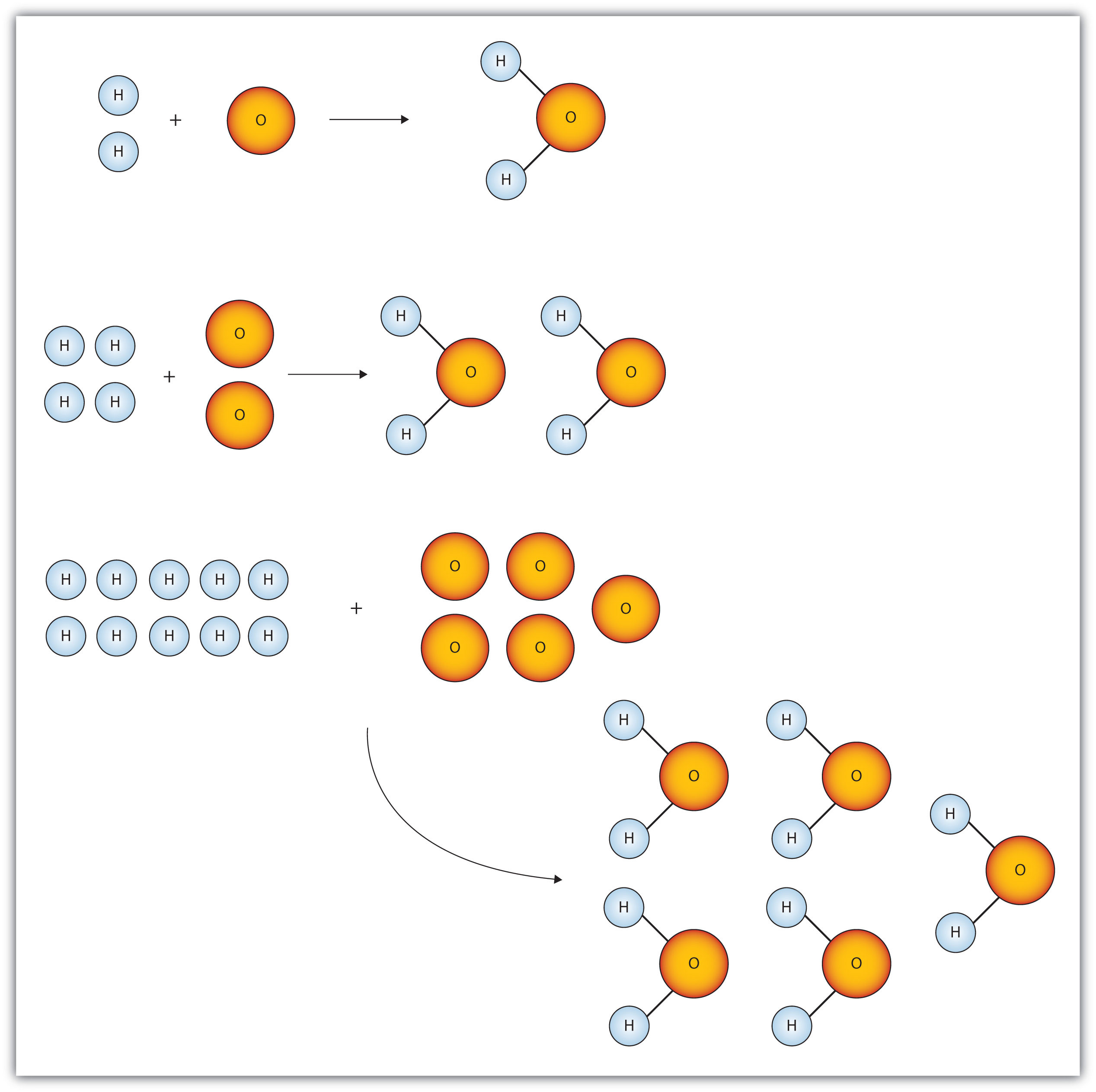
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent
CH104: Chapter 6 - Quantities in Chemical Reactions - Chemistry

PS GR 11 Session 11 LN
3g of H2 reacts with 29 g of O2 to give H2O.Find i) Limiting reagent ii)Max amount of H2O formed iii) Amount of reactants which remains unreacted.

Aqueous Transformation of a Metal Diformate to a Metal Dihydride Carbonyl Complex Accompanied by H2 Evolution from the Formato Ligands

Chapter 3 Chemical Reactions and Reaction Stoichiometry - ppt download

52. 80 g of H, is reacted with 80 g of O, to form water. Find out the mass of water obtained. Which substance is the limiting reagent?

US10328082B2 - Methods of use and combinations - Google Patents
How many grams of water can be produced if sufficient hydrogen reacts with 26.0g of oxygen? - Quora

Formic acid as renewable reagent and product in biomass upgrading - Tetrahedron Green Chem

Review of the Decomposition of Ammonia to Generate Hydrogen

van Geel text 2012.pdf - dvg
PPT - Mass Relationships in Chemical Reactions PowerPoint Presentation - ID:3181342

80 g of H2 is reacted with 80 g of O2 to form water. find out the mass of water obtained.which substance is

Hint : N2(g)+3H2(g)⟶2NH3(g) 28. 80 g of H2 is reacted with 80 g of O2..