The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is
By A Mystery Man Writer

Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor compressibility factor for onemole of a van der waals gas at 0c
Click here👆to get an answer to your question ✍️ The compression factor -compressibility factor- one mole of a van der Waals gas 0-C and 100 atm pressure is found to be 0-5- Assuming that the volume of a gas molecule is negligible- calculate the van der Waals- constant a
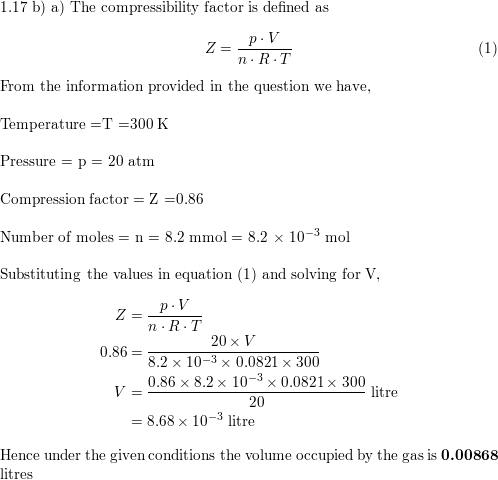
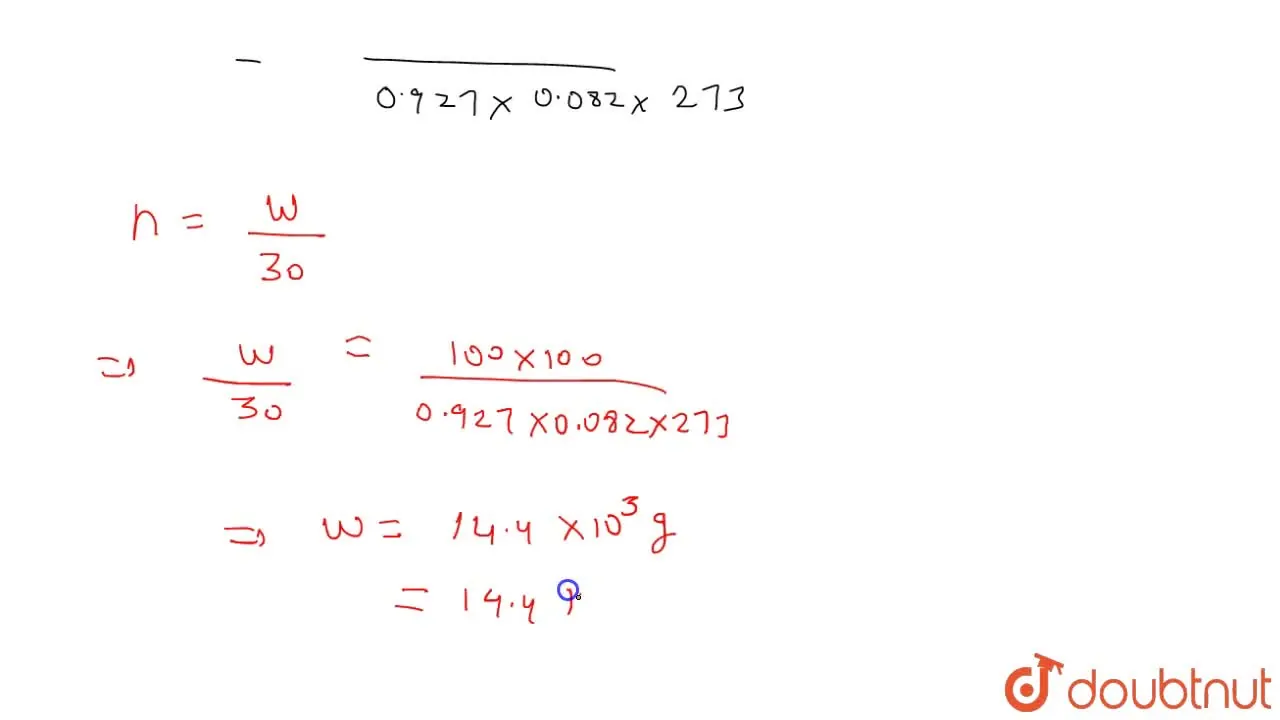
The compressibility factor for a given real gas is 0.927 at 273 K and

Pick only the incorrect statement.for gas A, a=0,the compressibility factor is linearly dependent on pressure.for gas C,aneq 0,bneq 0,it can be used to calculate a and b by giving lowest P value.for

The compression factor (compressibility factor) one mole of a van der Waals' gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is
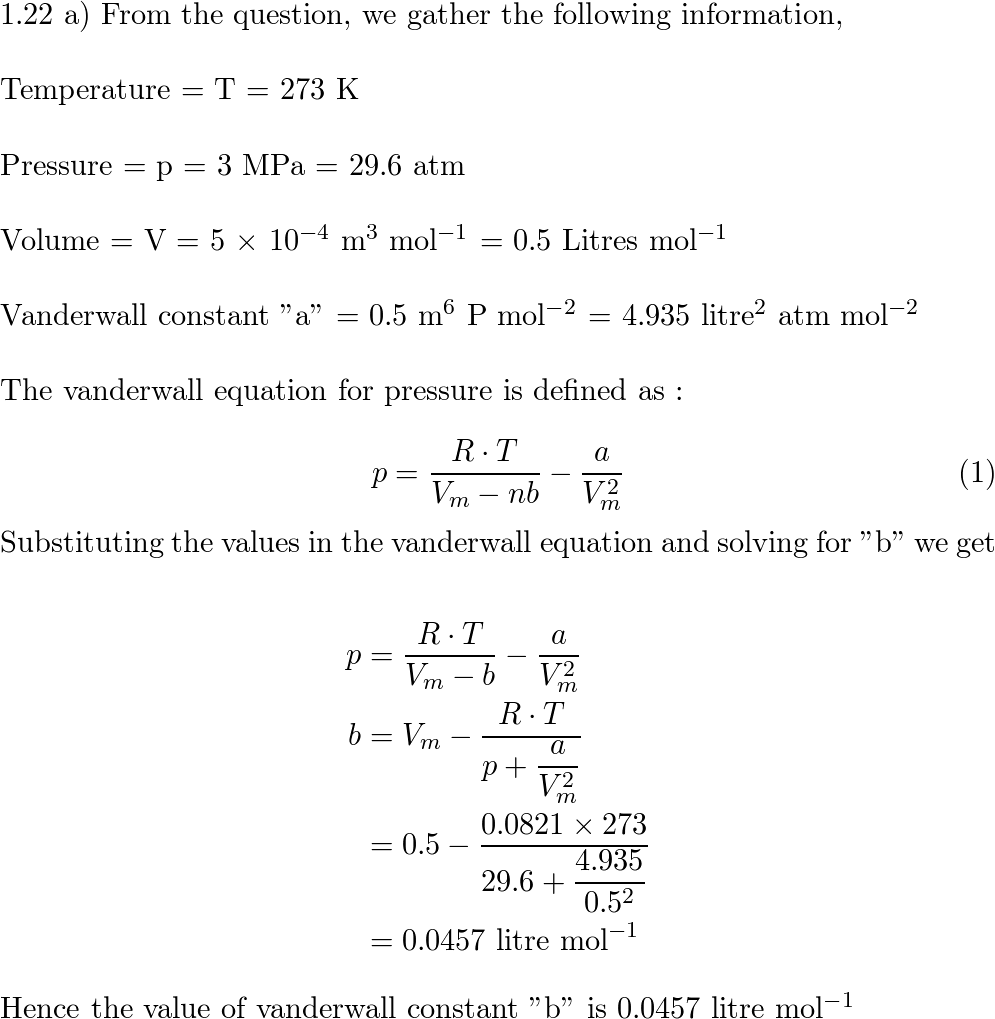
a) A certain gas obeys the van der Waals equation with $a =

The compression factor (compressibility factor) for one mole of a v

If `Z` is a compressibility factor, van der Waals' equation at low
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

Solved Question 1) For water at 293 K and 1 atm, the

Bengali] What will the value of compressibility factor (Z) be for a g

The compression factor (compressibility factor) for one mole of a Van der..

Van der Waals constant 'b' of a gas is 1250 litre/mole. How near

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at `0^()C` an

18. The compressibility factor one mole of a vanderwaal's gas 0°C and 100 atm pressure is found to be 0.5. Assume that the volume of gas molecule is negligible calculate the vanderwaals
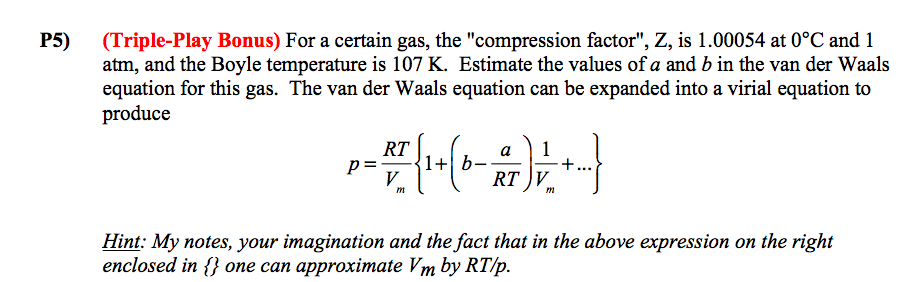
Solved (Triple-Play Bonus) For a certain gas, the