2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1.90 and 200 atm is 1.10.A certain mass of Noccupies a volume of 1
By A Mystery Man Writer

Click here:point_up_2:to get an answer to your question :writing_hand:2 112153 1215 jals 42 5the compressibility factor for nitrogen at 330 k and 800
Click here👆to get an answer to your question ✍️ -2- 1-12-15 -3- 12-15- Jals -4- 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1-90 and 200 atm is 1-10-A certain mass of Noccupies a volume of 1 dmat 330 Kand eoo atm calculate volume occupied by same cuany of gas 750 K and 200 atm- -1- 1 L -2- 2L -3- 3L

The compressibility factor for nitrogen at 330 K and 800 atm is
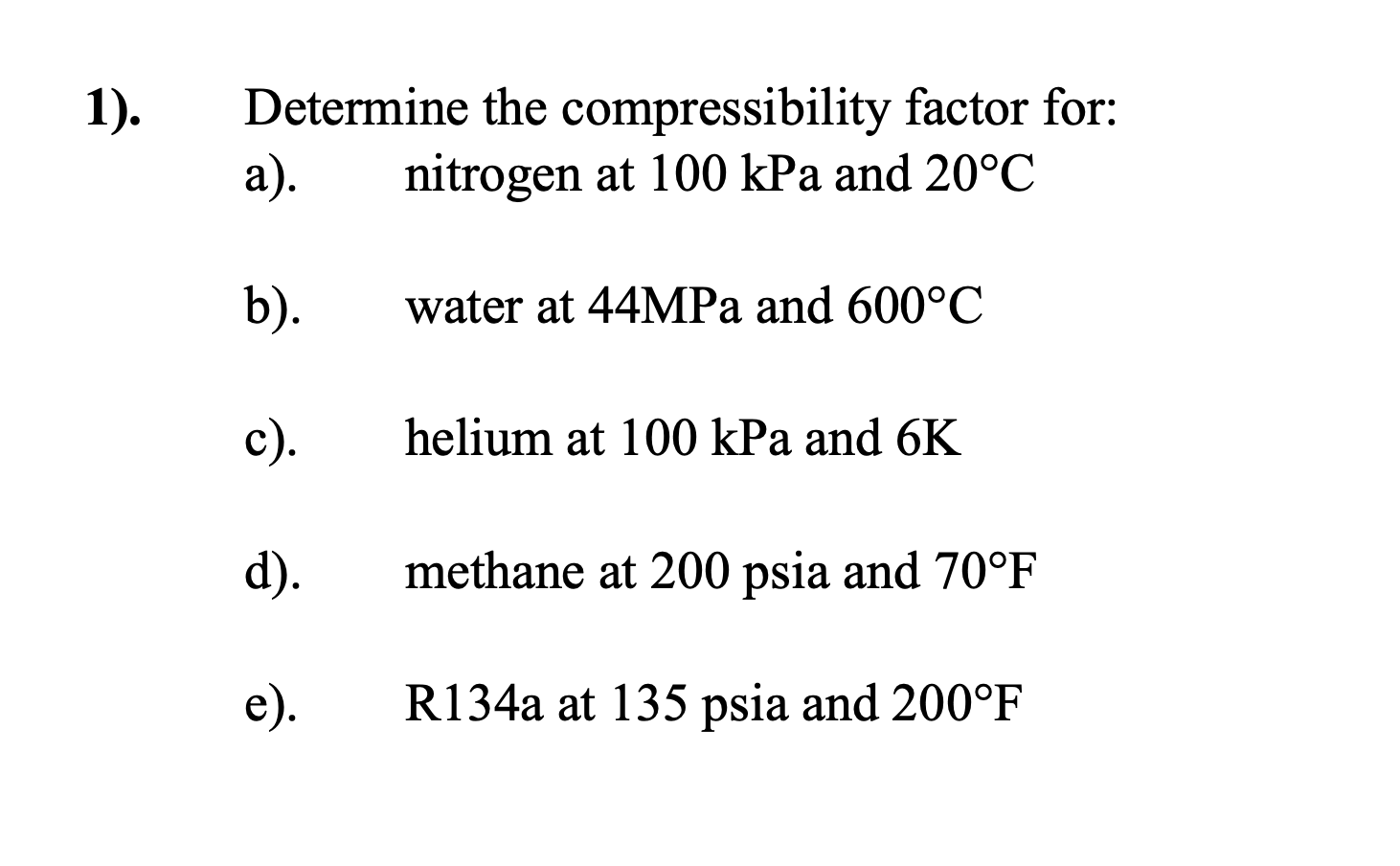
Solved Determine the compressibility factor for: a).

the compression factor one mole of a vander waals gas 0 C and 100

At total pressure P_1 atm and P_2 atm N_2O_4 is dissociated to an

Compressibility factor Z for N 2 at 23∘ C and 821 atm is 1.25

Advanced Thermodynamics Note 1 The 1st law and other basic

Calculate the compressibility factor for a gas, if 1 mole of it

3.3: Real gas and compressibility factor - Engineering LibreTexts
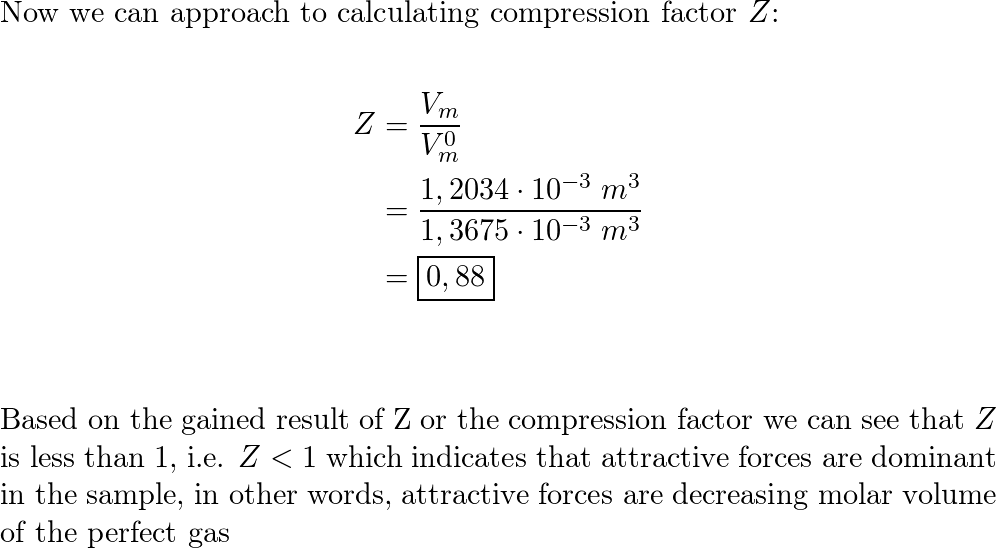
a) A gas at 250 K and 15 atm has a molar volume 12 per cent

Comperessibility factor (Z) for N(2) at -50^(@) C and 800 atm pressure
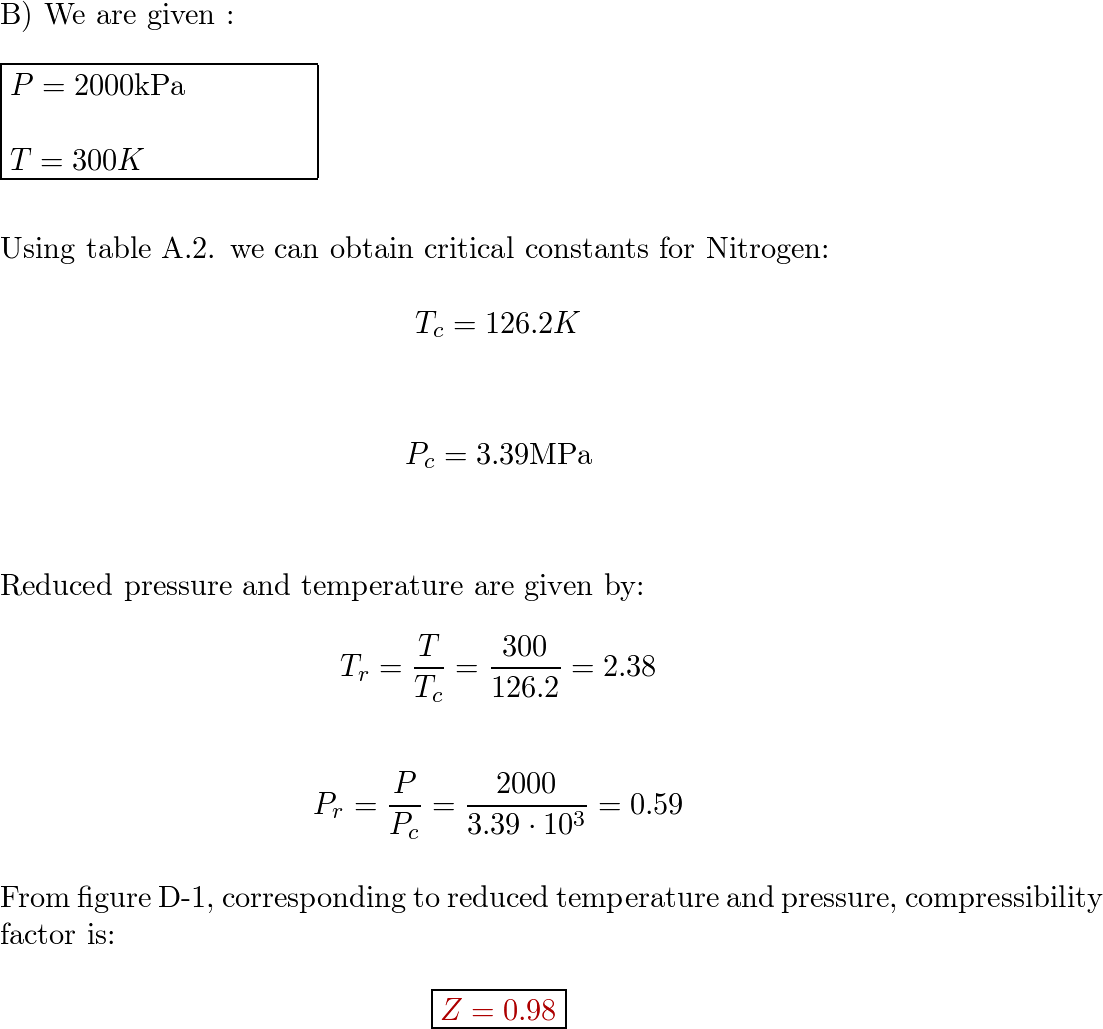
Find the compressibility factor for nitrogen at. 2000 kPa, 1
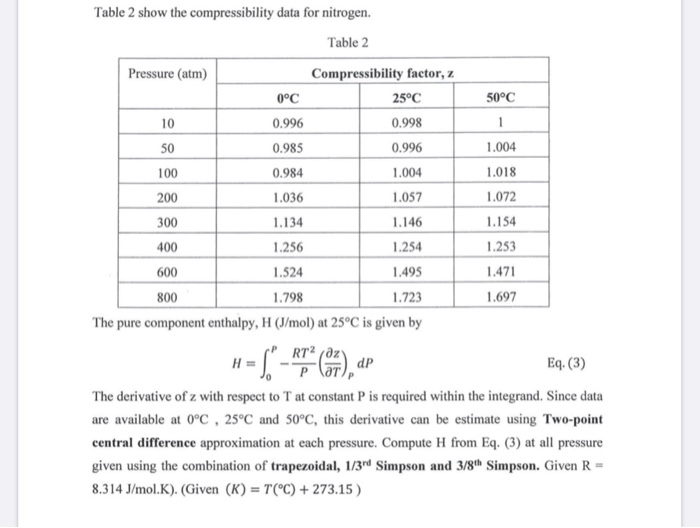
Solved Table 2 show the compressibility data for nitrogen.
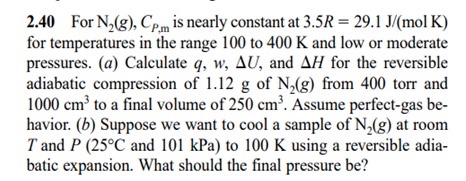
Solved For N2(g), CP,m is nearly constant at 3.5R = 29.1
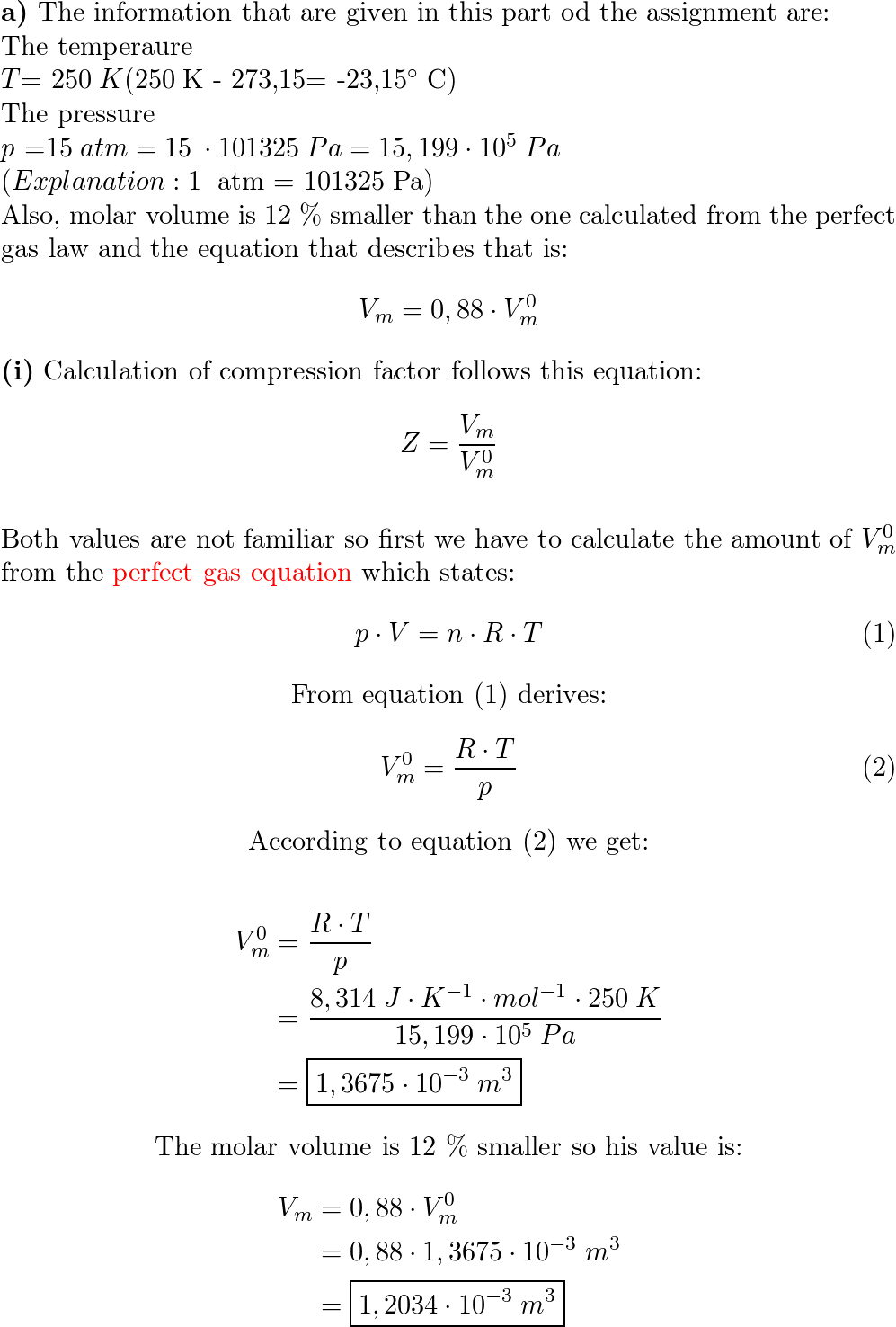
a) A gas at 250 K and 15 atm has a molar volume 12 per cent

The comperessibility factor for N(2) at -50^(@) C and 800 atm pressur