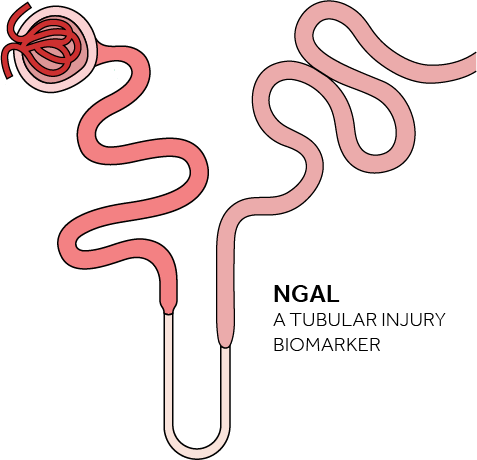
The NGAL Test is a particle-enhanced turbidimetric immunoassay for the quantitative determination of NGAL in human urine and plasma on automated clinical chemistry analyzers. NGAL measurements are useful in the risk assesment of AKI.

Elevated Neutrophil Gelatinase-Associated Lipocalin Is Associated With the Severity of Kidney Injury and Poor Prognosis of Patients With COVID-19 - ScienceDirect

Identification of Urinary Activin A as a Novel Biomarker Reflecting the Severity of Acute Kidney Injury

BioPorto Diagnostics A/S

PDF) Cost-effectiveness and value of information analysis of NephroCheck and NGAL tests compared to standard care for the diagnosis of acute kidney injury

Ulcer-associated cell lineage expresses genes involved in regeneration and is hallmarked by high neutrophil gelatinase-associated lipocalin (NGAL) levels. - Abstract - Europe PMC

Prioritised areas for future research

NGAL - Bioporto
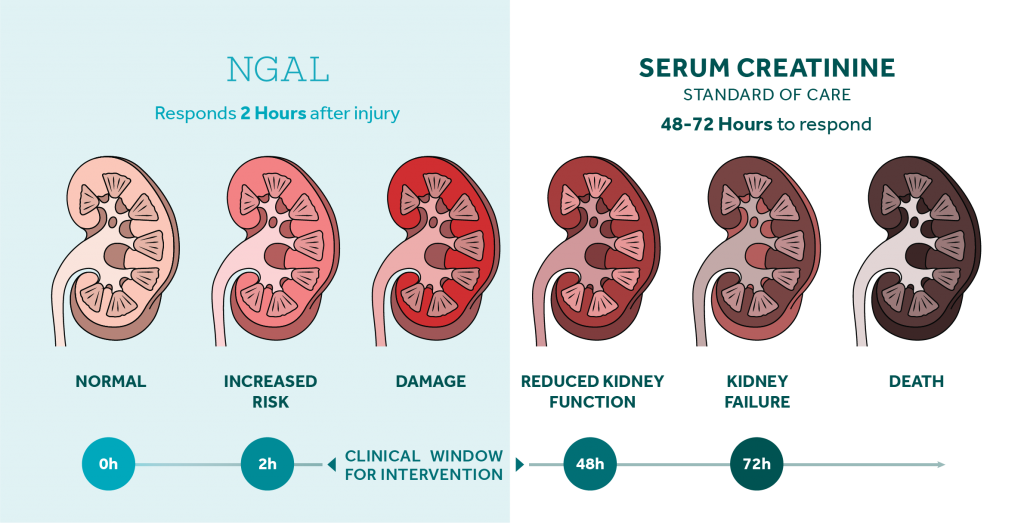
NGAL - Bioporto
BioPorto Submits Application for Marketing Authorization of NGAL Test to the US Food and Drug Administration
Dog NGAL ELISA Kit - Bioporto