If Z is a compressibility factor, van der Waals equation at low
By A Mystery Man Writer

Solution For If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 1: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 2: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 3: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 4: If Z is a compressibility factor, van der Waals equation at low pressure can be written as

Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced

If Z is a compressibility factor, van der Waals equation at low
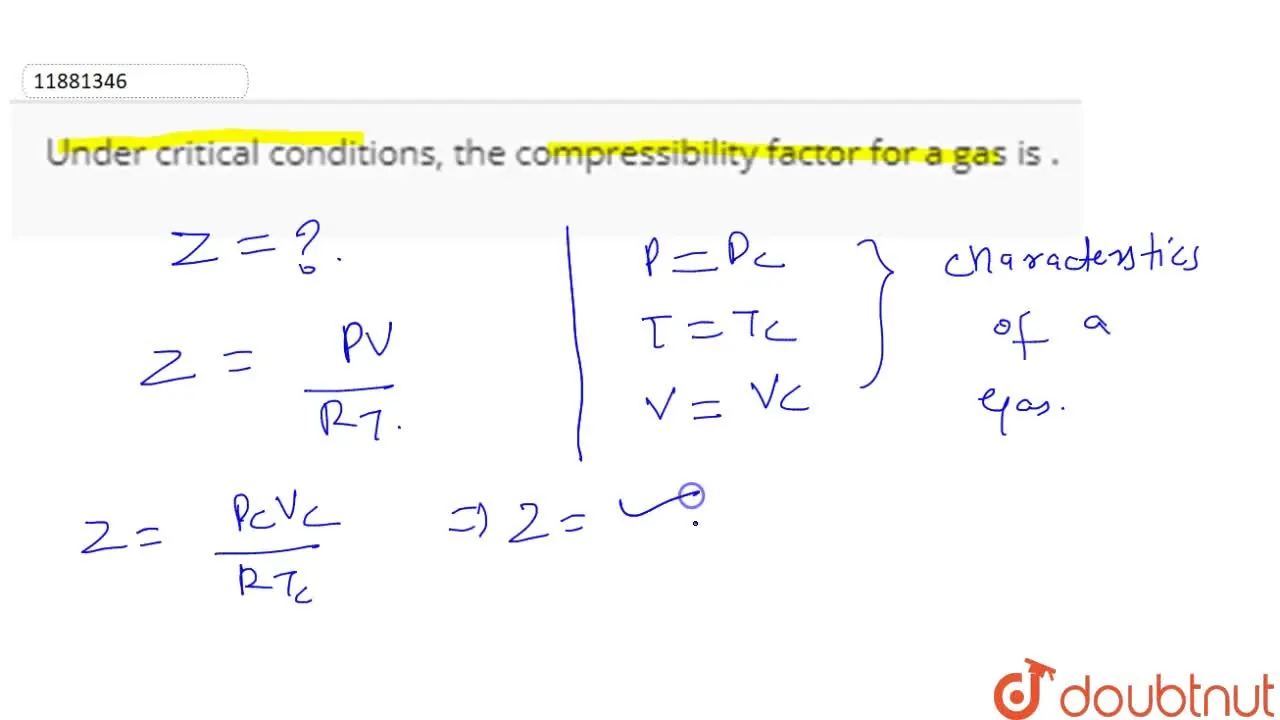
Under critical conditions, the compressibility factor for a gas is .
How to calculate the values of critical pressure and temperature for a given gas (Van der Waals equation) - Quora

If `Z` is a compressibility factor, van der Waals' equation at low
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to
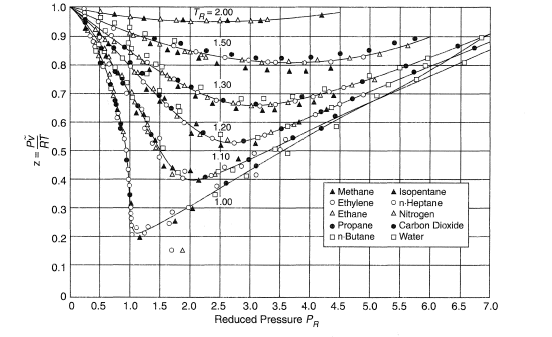
GAS LAW
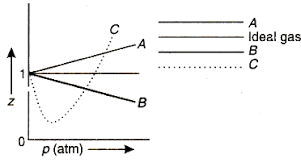
For one mole of a van der Waals gas when b0andT300K the PVvs1Vplot

Compressibility factor (gases) - Citizendium

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

If `Z` is a compressibility factor, van der Waals' equation at low