Bond length of H H is 0.64 and the bind length of F2 is 1.2. Electronegativities of H and F respectively are 2.1 and 4.1.What is the bond length of HF? 1)0.64 2)0.92 3)0.82 4)0.62
Bond length of H-H is 0-64 and the bind length of F2 is 1-2- Electronegativities of H and F respectively are 2-1 and 4-1-What is the bond length of HF- 1-0-64 2-0-92 3-0-82 4-0-62

Solved The electronegativity is 2.1 for H and 4.0 for F.
What is the change in bond length of hetronuclear molecules due to difference of electronegativity value of bonded atom? - Quora

Chapter 1-5 PDF, PDF, Neutron

Actinides - Chemistry and Phys. Properties - Structure & Bonding v.59 (1985) Pp.1-298, PDF, Electron Configuration

Phosphorescent organic light-emitting devices: Iridium based emitter materials – An overview - ScienceDirect

Welcome to Chem Zipper.com: Why N-N bond length of H2N-NH2 is greater than N-N bond length of F2N-NF2 molecules?

Which of the following has the minimum bond length ?
Why does H-F being more electronegative than H-Cl cause it to have a stronger bond? - Quora
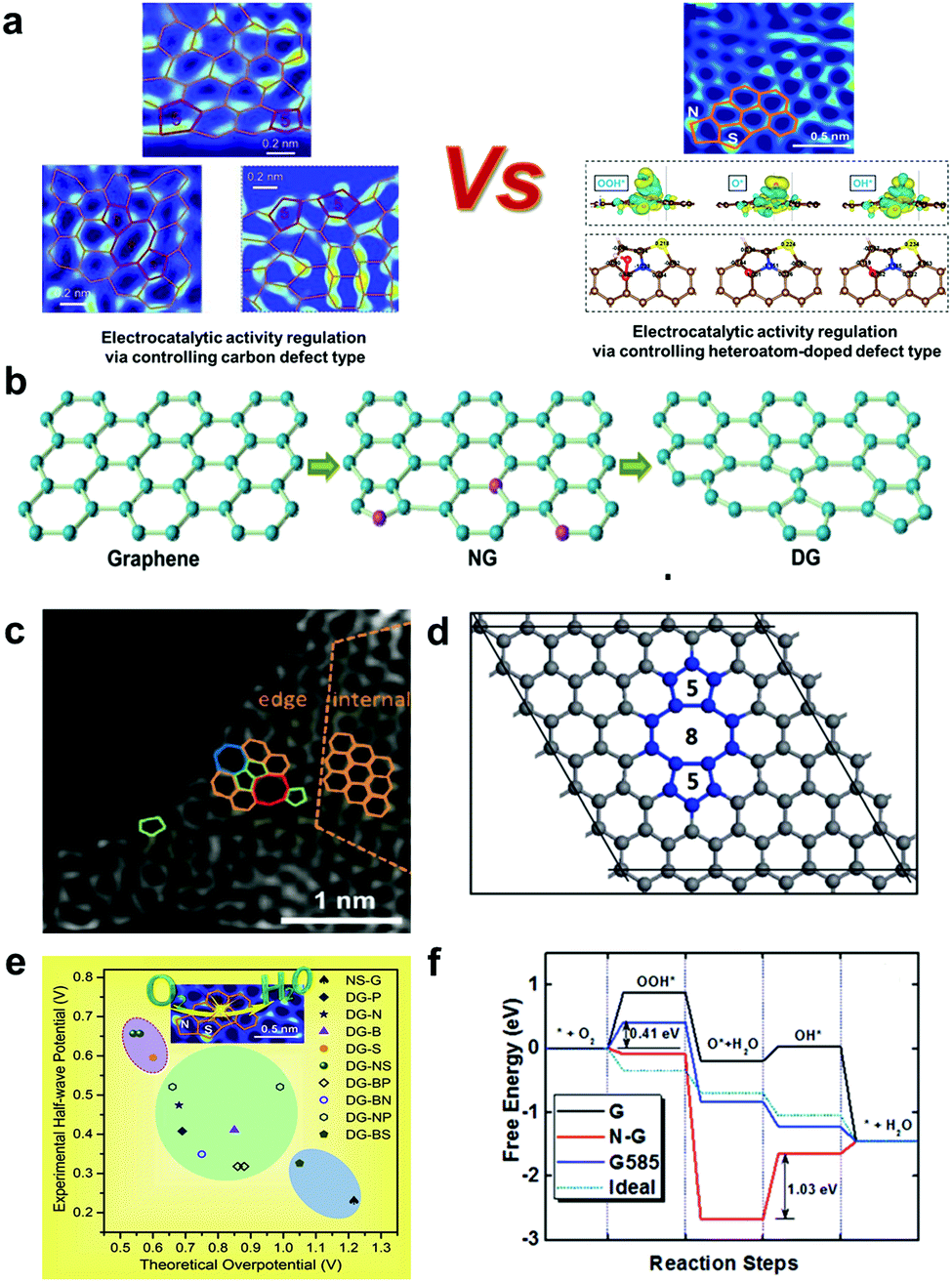
Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials - Energy & Environmental Science (RSC Publishing) DOI:10.1039/D1EE00166C

WO2015057963A1 - Fgfr4 inhibitors - Google Patents

Chapter 1-5 PDF, PDF, Neutron

Oxygen reduction electrochemistry at F doped carbons: A review on the effect of highly polarized C-F bonding in catalysis and stability of fuel cell catalysts - ScienceDirect