Pick only the incorrect statement.for gas A, a=0,the compressibility factor is linearly dependent on pressure.for gas C,aneq 0,bneq 0,it can be used to calculate a and b by giving lowest P value.for
By A Mystery Man Writer

Click here:point_up_2:to get an answer to your question :writing_hand:pick only the incorrect statement
Click here👆to get an answer to your question ✍️ Pick only the incorrect statement-for gas A- a-0-the compressibility factor is linearly dependent on pressure-for gas C-aneq 0-bneq 0-it can be used to calculate a and b by giving lowest P value-for gas B-0-if b-0-the compressibility factor is lineraly dependent on pressure-slope all three gases high pressure is positive
Solution- -C-xA0-for gas C-a-x2260-0-b-x2260-0- it can be used to calculate a and b by giving lowest P value-According to the real gas equation-The constants -apos-a-apos- and -apos-b-apos- are Van der Waals constant for attraction and volume for a given gas-The -apos-a-apos- values for a given gas are measure of intermolecular forces of attraction- More are the intermolecular forces of attraction- more will be the value of a-xA0-For a given gas van der Waals constant of attraction -apos-a-apos- is always greater than van der Waals constant of volume -apos-b-apos-xA0-The gas having higher value of -apos-a-apos-xA0- can be liquefied easily and therefore H2 and He are not liquefied easily-According to this- for gas A-Z-gt-1-a-0 and its dependence on P is linear at all pressure and for gas B-Z-lt-1-b-0 and its dependence on P is linear at all pressure-Also- at high pressure- the slope is positive for all real gases
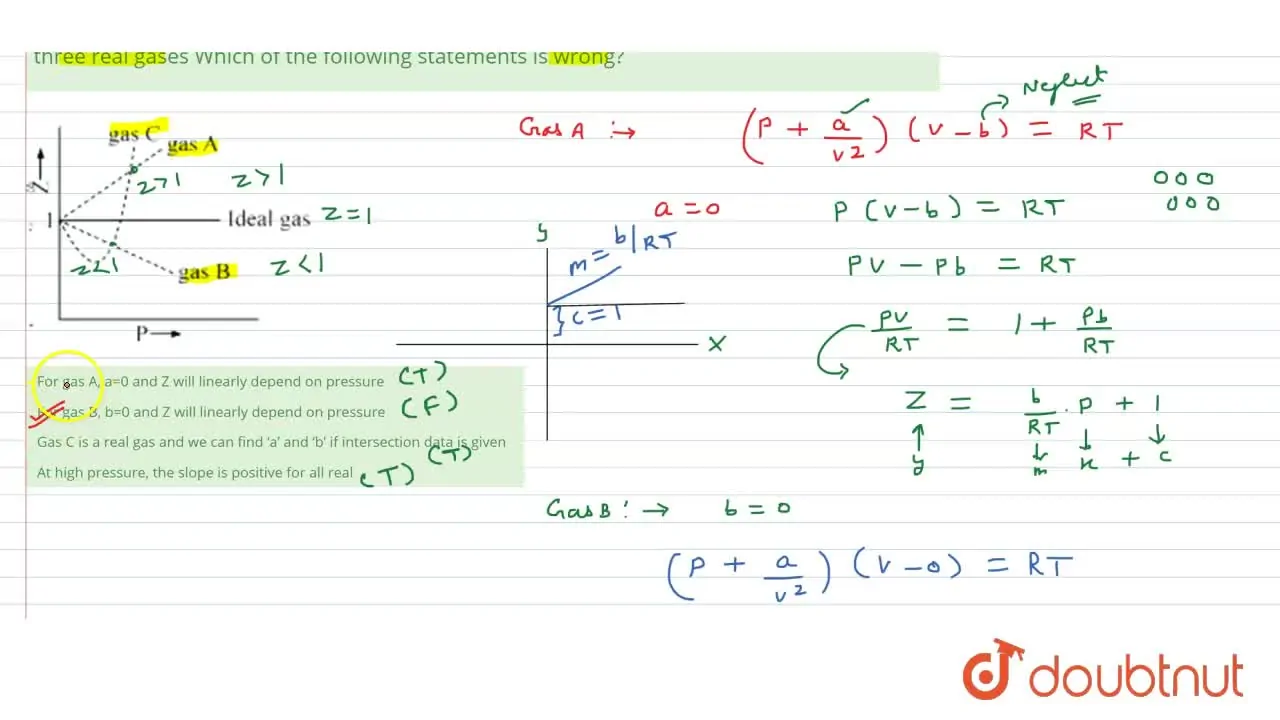
Gas C is a real gas and we can find 'a' and 'b' if intersection data i

Solved Show that if the compressibility factor is given by Z
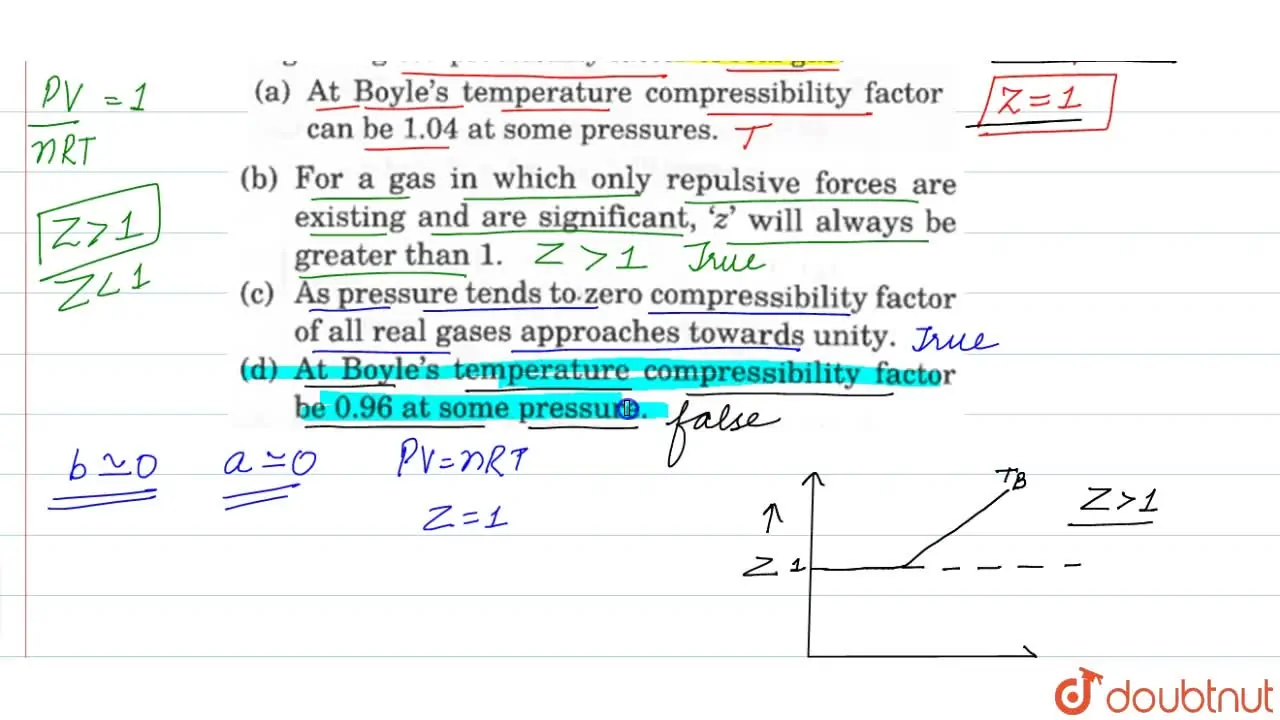
As pressure tends to zero compressibility factor of all real gases app
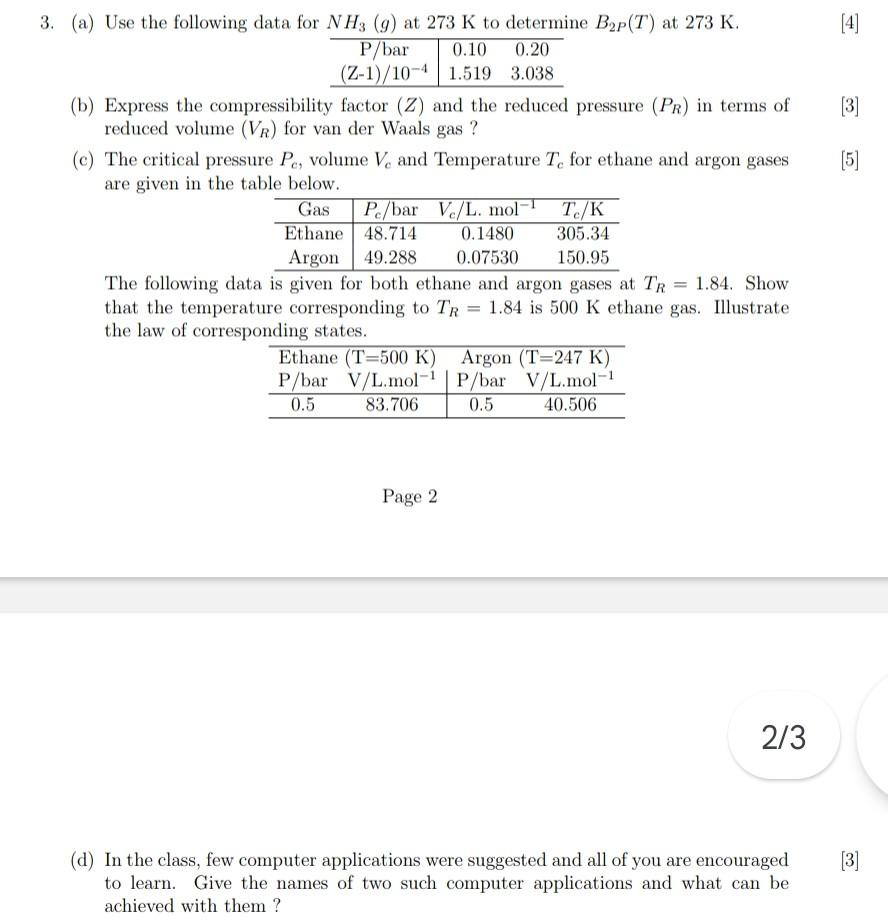
Solved (b) Express the compressibility factor (Z) and the

Solved Van der Waals equation is given below: P = ( nRT, )
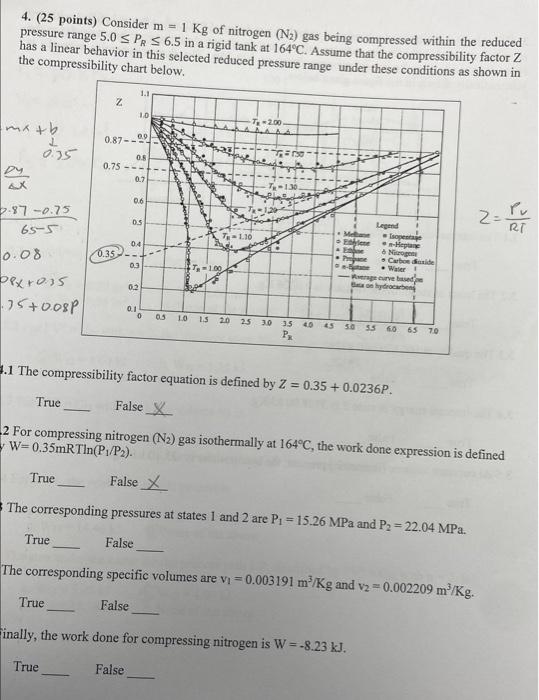
Solved 4. (25 points) Consider m=1Kg of nitrogen (N2) gas

Solved Experiment1 Experiment 3 In the laboratory, a

Liquid 116. Which of the following is the only incorrect statement - Ideal gas X L (1) For the gas (A): 0 and it varies linearly with P Hut2) For the gas (

Knjiga, PDF, Fluid Dynamics

variations of 2 12.7 (a) eb (c)-(ar (d) - 6. The given graph represent the variations (compressibility factor (Z)=- gases A, B and C. Identify the only incorrect statement pl) versus p

Solved The compression factor (Z) for a real gas can be