The compression factor (compressibility factor) for 1 mol of a van der
By A Mystery Man Writer

For 1 mol of a gas, the van der Waals equation is (P+(a)/(V(m)^(2)))(V(m)-b)=RT Ignoring b, we get (given volume of gas molecule is negligible) (P+(a)/(V(m)^(2)))V(m)=RT ltbgt or pV(m)+(a)/(V(m))=RT or (pV(m))/(RT)+(a)/(V(m)RT)=1 or Z=(pV(m))/(RT)=1-(a)/(V(m)RT) (i) It is given that Z=(pV(m))/(RT)=0.5implies V(m)=(0.5RT)/(P) With this, equation (i) becomes 0.5=1-(a)/((0.5RT//p)RT) or a=(0.5)((0.5RT)/(p))RT=0.25(R^(2)T^(2))/(p) Substiuting the given values, we get a=(0.25)[((0.082L atm K^(-1)mol^(-1))^(2)(273 K)^(2))/((100 atm))] =1.2528 L^(2) atm mol^(-2)

The compressibility factor for definite amount of van der Waals' gas a
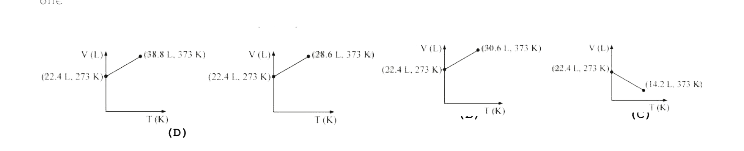
The compressibility factor of gases is less than unity at STP. Therefo
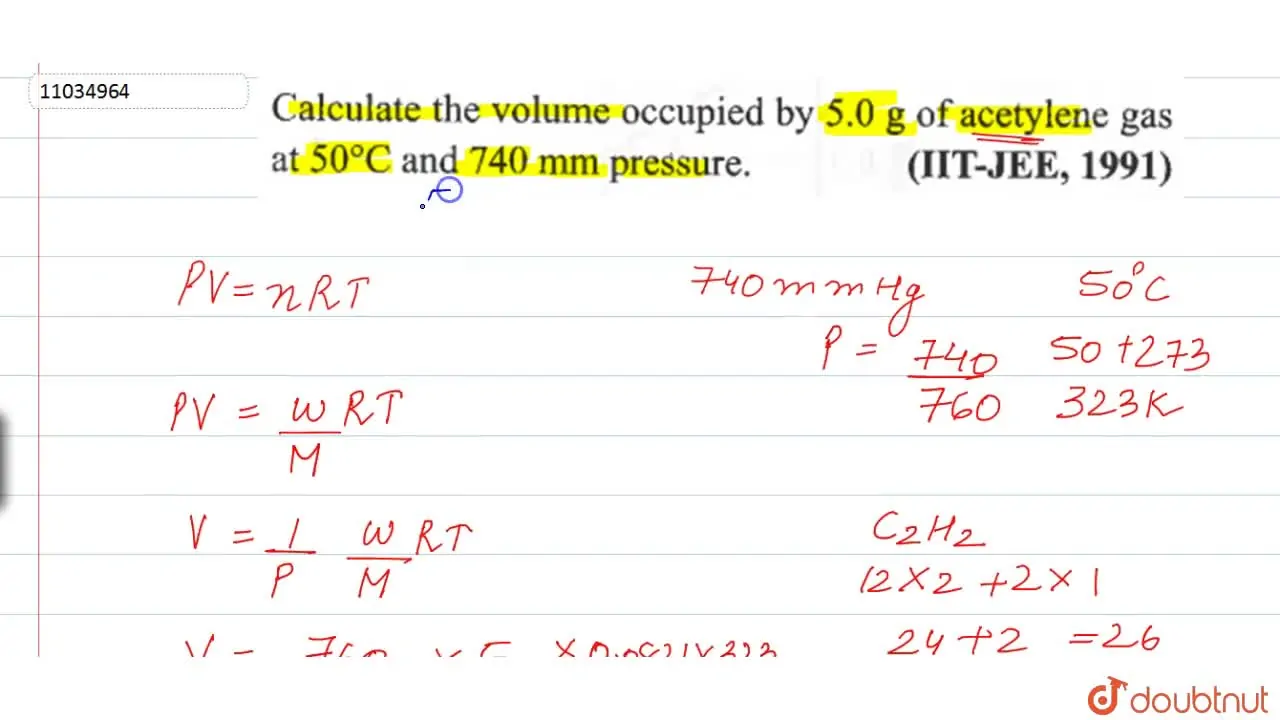
Calculate the volume occupied by 5.0 g of acetylene gas at 50^(@)C and
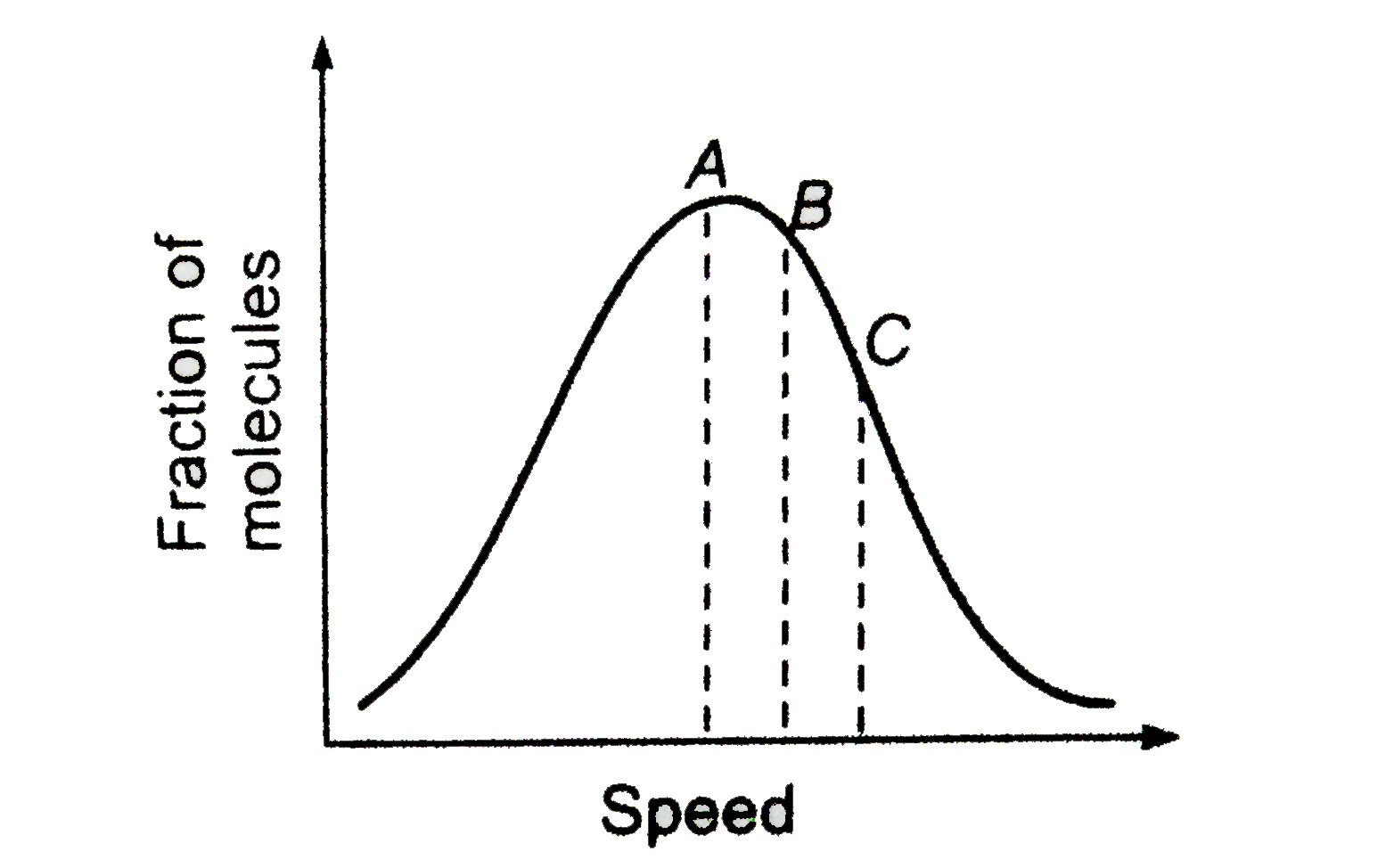
Distribution of molecules with velocity is represented by the curve
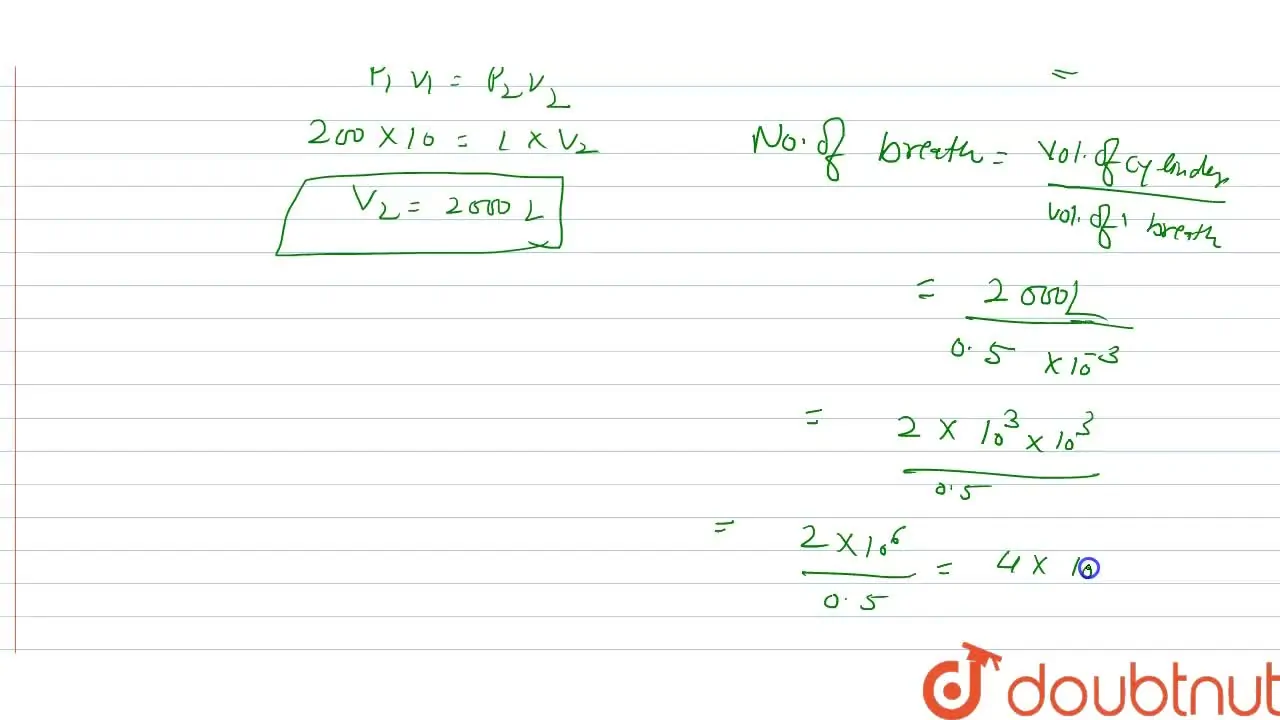
In a hospital, an oxygen cylinder holds 10 L of oxygen at 200 atm pres
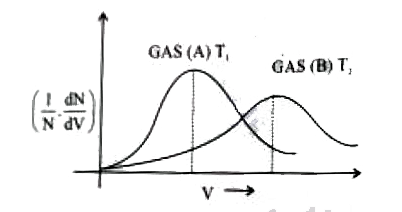
For two gases A and B,P v//s V isotherms are drawn at T K as shown, T
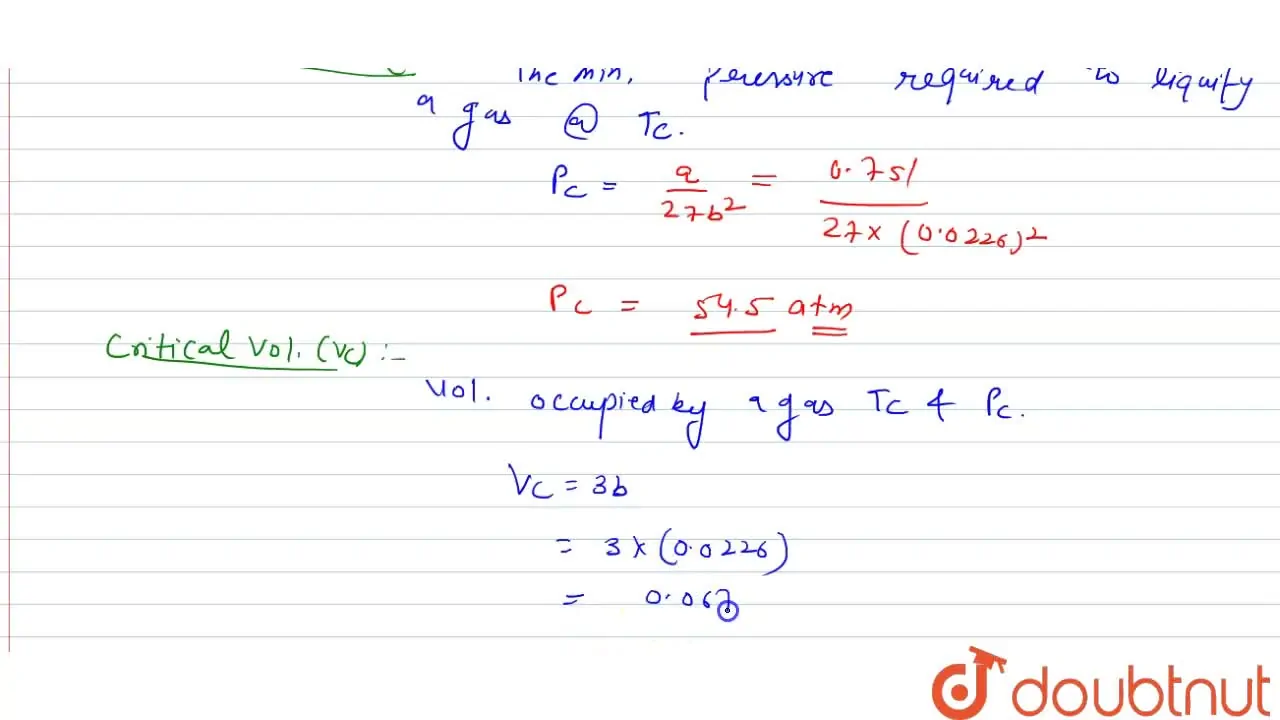
Calculate the critical constants of a gas whose van der Waals constant
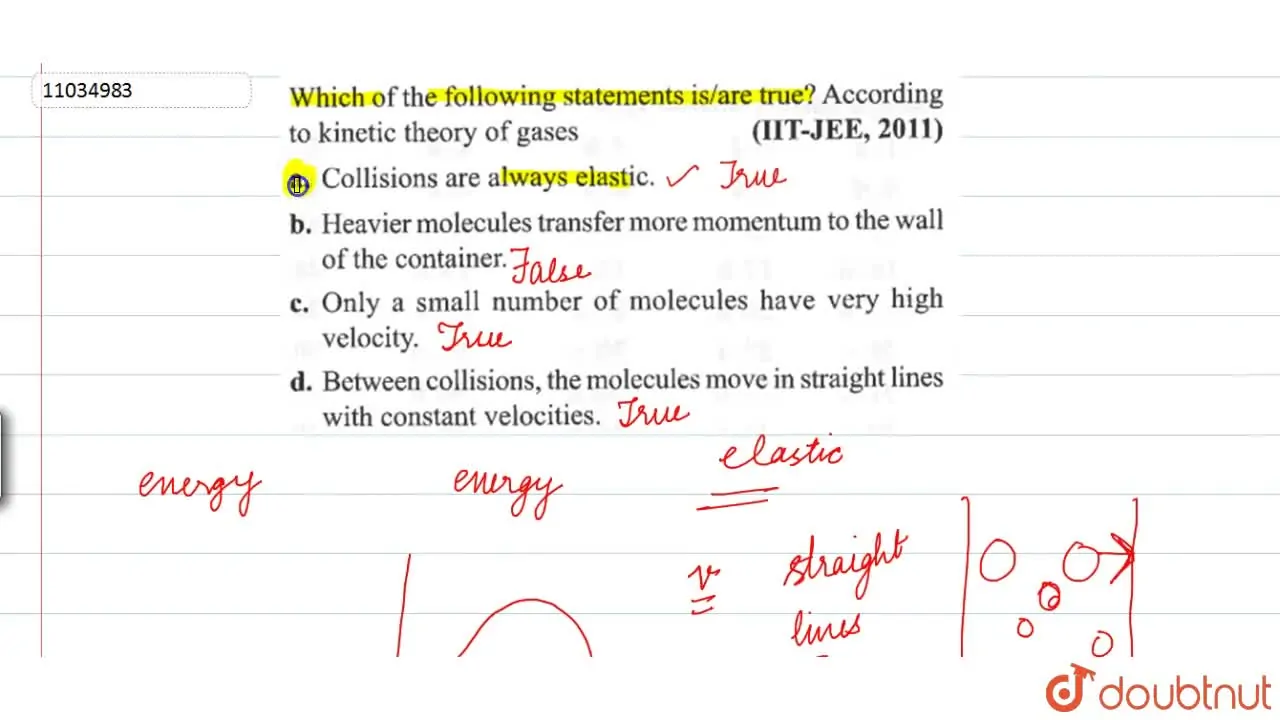
Only a small number of molecules have very high velocity.

Which of the following equations represents the compressibility factor