Consider the graph between compressibility factor Z and pressure P
By A Mystery Man Writer
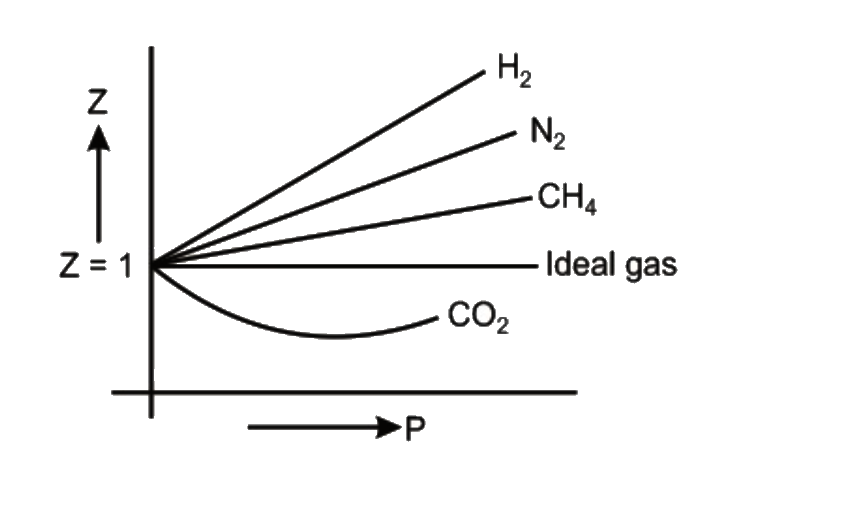
Consider the graph between compressibility factor Z and pressure P, The correct increaing order of ease of liquefaction of the gases shown in the above g
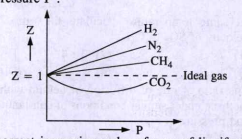
Consider a graph between compressibility factor Z and pressure P

physical chemistry - Is the compressibility factor smaller or
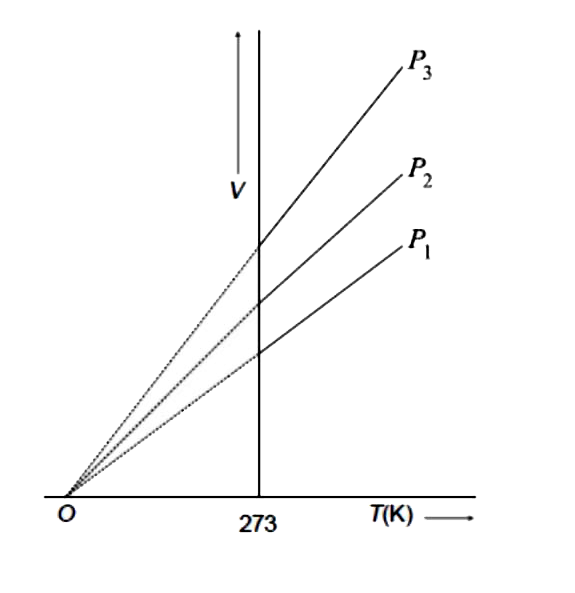
Consider the graph between compressibility factor Z and pressure P
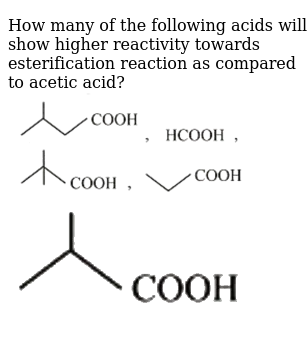
How many of the following acids will show higher reactivity towards es

The graph of compressibility factor (Z) vs. P for one mole of a

If the uncertainty in the position of a particle is equal to its de-Br

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

PV Compressibility factor Z= nRT is plotted against pressure : N

For a given gas, a graph is shown between compressibility factor

Consider the graph between compressibility factor Z and pressure P
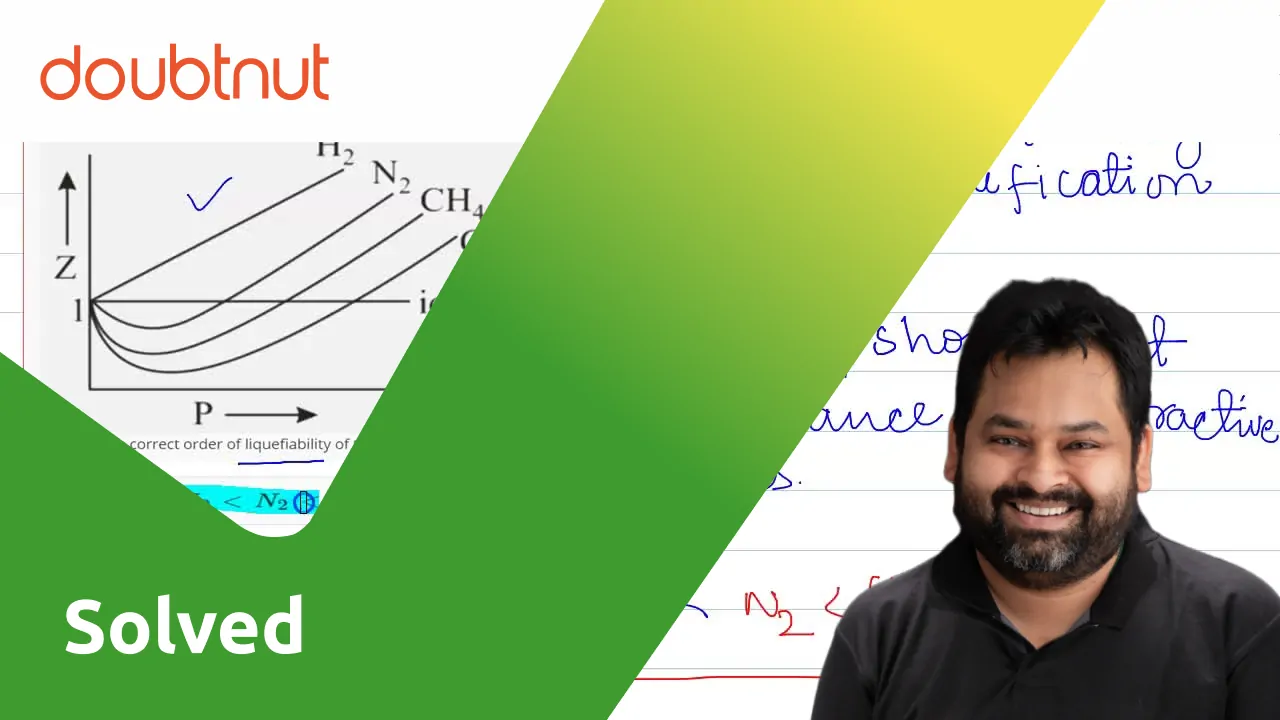
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
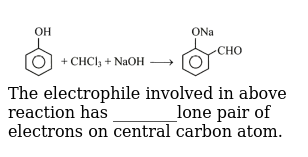
The electrophile involved in above reaction has lone pair of electrons

Energies, Free Full-Text
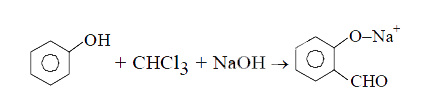
The electrophile involved in above reaction has lone pair of electrons