SOLVED: For a gas at a given temperature, the compression factor is described by the empirical equation: z = 1 - 8.50 × 10^(-3)P/P° + 3.50 × 10^(-5)(P/P°)^2 where P° = 1
By A Mystery Man Writer

VIDEO ANSWER: Hello students: let's look at the question: l n, that integrate integration and 0 z minus 1 bracket, close d p by p here. Minus 1 is equal to minus 8.50 into 10 to the power minus 3 p by p, not plus 3.50 into 10. To the power minus 9. P
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

OneClass: For a gas at a given temperature, the compression factor is described by the empirical equa

2006 Supplement to the Florida Building Code

SOLVED: Calculate the compressibility factor Z for propane at 115ºC and 10 bar using the virial-type equation truncated in the third term, with the values of B and C obtained with the

Ficoquimica, PDF, Gases

Fundamentals of Compressible Fluid Mechanics

2,500 Solved Problems in Fluid Mechanics and Hydraulics, PDF, Pressure

Viscoplastic Rheology of α‐Quartz Investigated by Nanoindentation - Strozewski - 2021 - Journal of Geophysical Research: Solid Earth - Wiley Online Library

PDF) Seismic Fragility of Buried Steel Natural Gas Pipelines due to Axial Compression at Geotechnical Discontinuities

Chemical engineering by Mauricio - Issuu
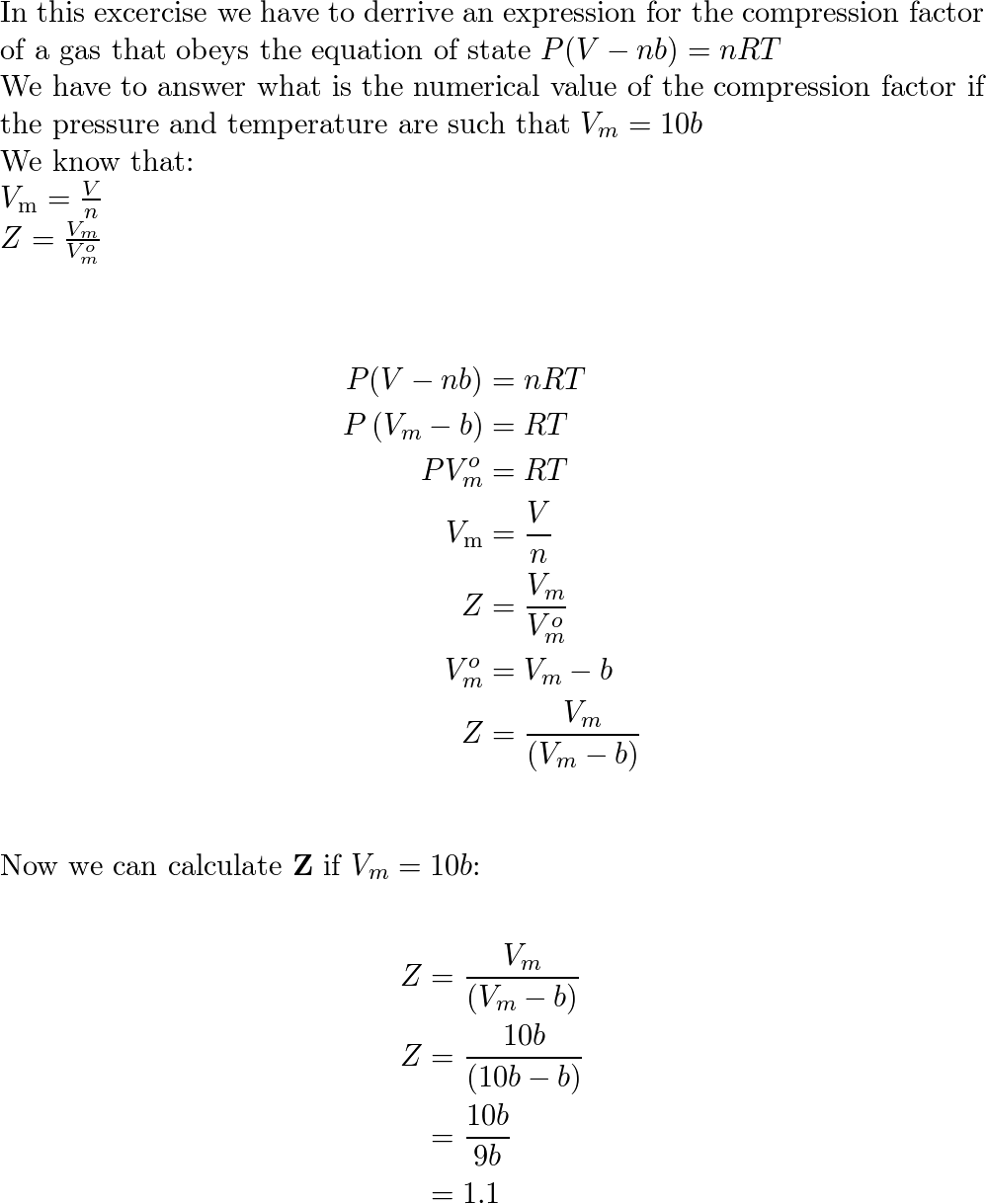
Derive an expression for the compression factor of a gas tha

Soil water diffusivity and water content distribution during outflow experiment
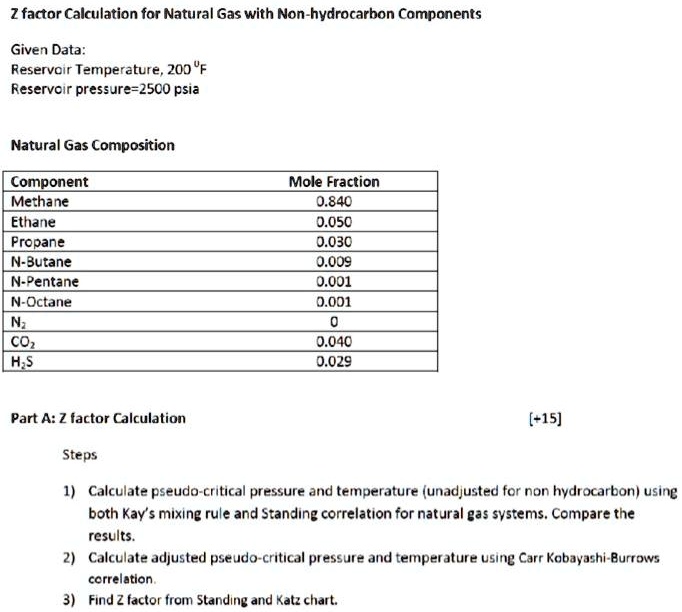
SOLVED: Question #2 only Z factor Calculation for Natural Gas with Non-hydrocarbon Components Given Data: Reservoir Temperature,200F Reservoir pressure=2500psia Natural Gas Composition Component Methane Ethane Propane N-Butane N-Pentane N-Octane N CO HS