
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC), including those with brain metastases. The update follows last year’s accelerated approval of the drug for people with TNBC.
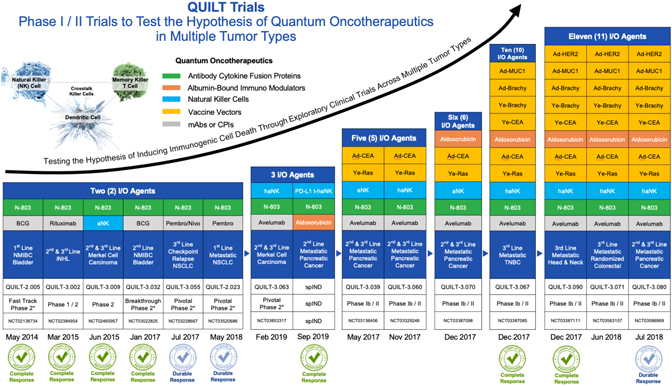
EX-99.2
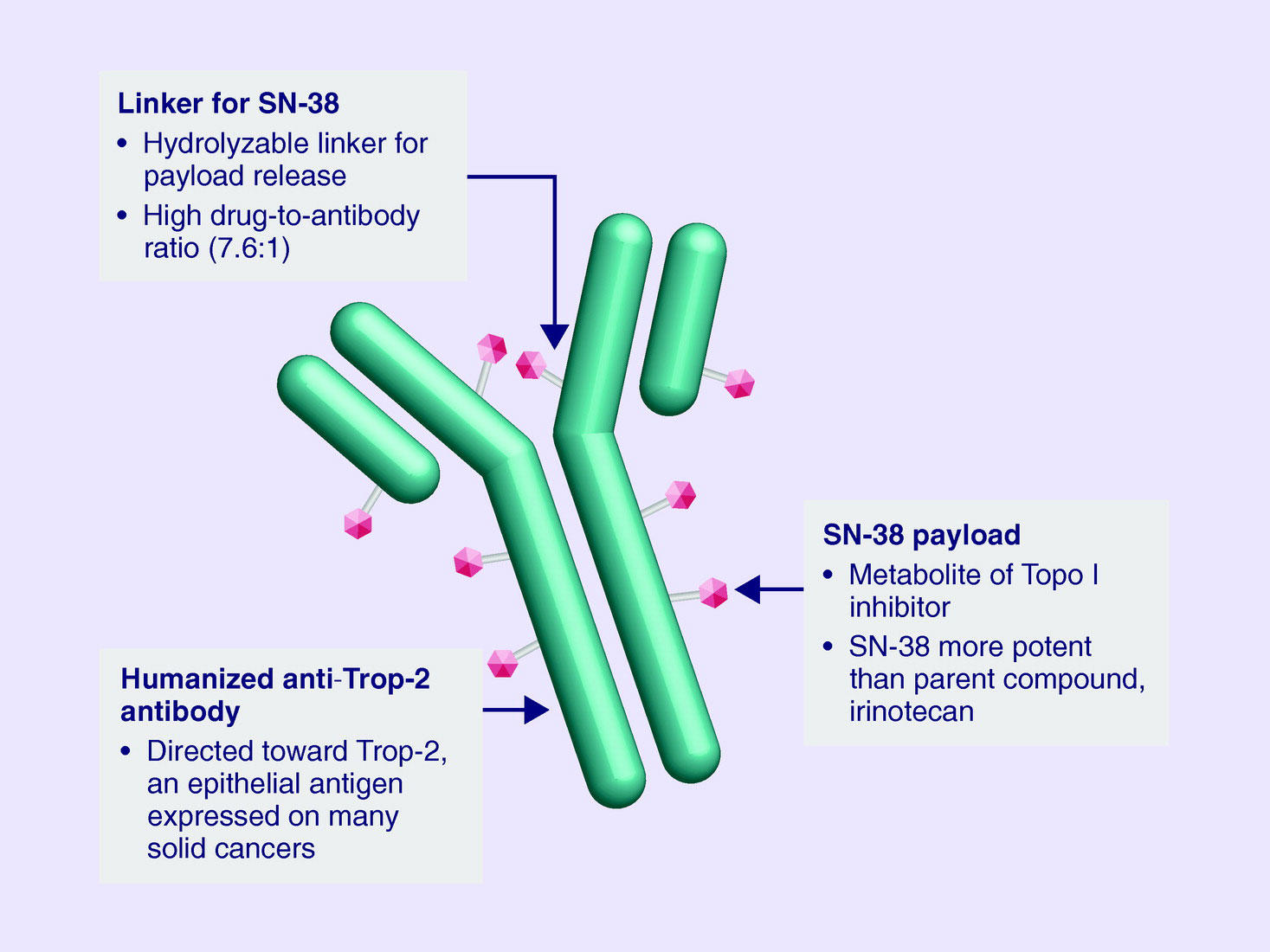
Sacituzumab Govitecan for Metastatic Triple-Negative Breast Cancer

Another Player in Advanced Bladder Cancer

Kevin Punie on X: #ESMO21 Sacituzumab govitecan is a major

Sacituzumab Earns Regular FDA Approval For TNBC NCI
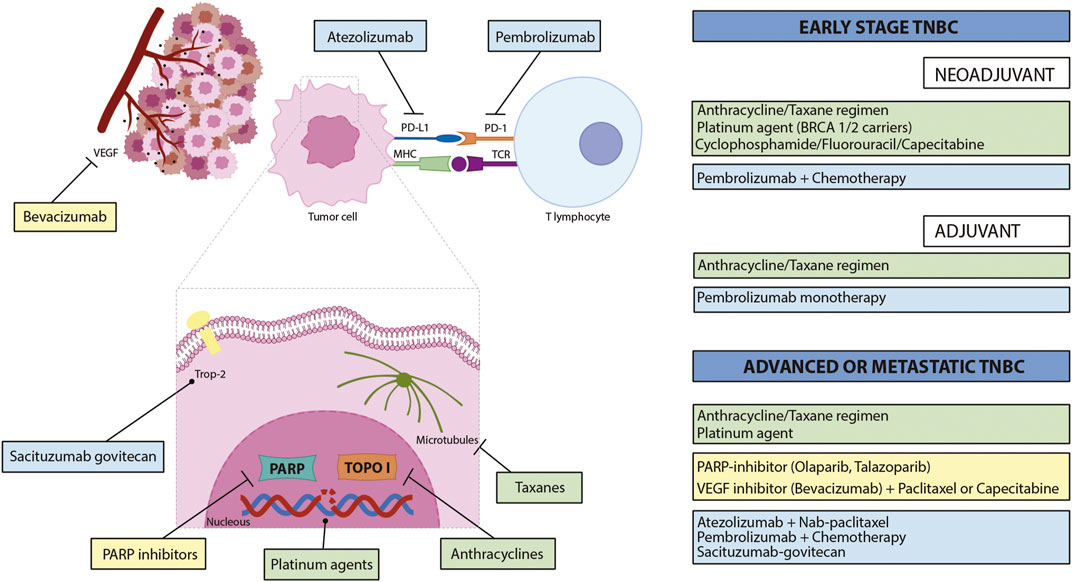
Frontiers Immunotherapy in triple-negative breast cancer

Mission Mountain Wilderness

Pharmaceutics, Free Full-Text

Mission Mountain Wilderness